Introduction
Overactive bladder (OAB) is a disease that affects physical functioning and social life and significantly decreases quality of life. According to the International Continence Society definition, OAB consists of urinary urgency with or without urge incontinence, often accompanied by frequency and nocturia. OAB was estimated to affect 20% of the world population in 2018 [1]. Current guidelines recommend antimuscarinic drugs as a mainstay of management of OAB [2]. Side effects of antimuscarinic agents occur in a high percentage of patients and may lead to withdrawal of treatment [3]. Adverse effects commonly include dry mouth and constipation [4]. Moreover, changes in blood pressure, pulse rate, or ECG may occur, and these effects may be important in patients with hypertension [4]. Therefore, a search for new drugs with fewer side effects is justified.
In June 2012, the FDA approved mirabegron as a new therapeutic approach to OAB. Mirabegron is a selective agonist at β3-adrenergic receptor (β3-AR), which activates the β3-AR in the urinary bladder, resulting in its relaxation [5]. Although mirabegron is a well-tolerated alternative to antimuscarinic drugs, it also affects the cardiovascular system by stimulation of β1-adrenergic receptor (β1-AR) in the heart. It has been shown that mirabegron acts on both β1-AR and β2-adrenergic receptor (β2-AR), increasing heart rate and blood pressure; therefore, it is not recommended for patients with severe uncontrolled hypertension [6].
Nebivolol (NEBI) is a potential novel treatment option for patients with OAB and concomitant hypertension. It is a cardioselective, lipophilic, third-generation β-blocker, which blocks β1-ARs in the heart and has a nitric oxide (NO) potentiating and vasodilatatory effect. NEBI is approved for hypertension and heart failure treatment [7]. Moreover, similarly to mirabegron, NEBI is an agonist of β3-ARs. Activation of β3-AR results in relaxation of the bladder and prevention of frequent urination [8]. That is why this drug seems to be a reasonable treatment option for OAB patients, especially those with concomitant hypertension [9].
The aim of this study was to assess the impact of NEBI on OAB symptoms in spontaneously hypertensive rats (an animal model of OAB and hypertension). Additionally, we investigated the influence of NEBI on mean arterial pressure (MAP), other cardiovascular parameters, and biochemical markers.
Material and methods
Study design
In the experiments 60 female rats, weighing 200–225 g, were divided into four experimental groups of 15 animals each: 1) Wistar-Kyoto rats, receiving physiological saline for 14 days (WHY), which were used as normotensive age-matched controls; 2) Spontaneously hypertensive rats receiving physiological saline for 14 days (SHR); 3) WHY plus nebivolol 0.05 mg/kg for 14 days (WHY + NEBI); 4) SHR plus nebivolol 0.05 mg/kg for 14 days (SHR + NEBI).
The animals were kept in environmentally controlled rooms in standard cages with unlimited access to aliment. All animals were experimentally naive and were tested one time only.
At the beginning of the experiment the bladder and arterial catheters were surgically inserted in all rats. Then NEBI (at a single daily dose of 0.05 mg/kg) or vehicle were administered intra-arterially via a polyethylene catheter inserted into the carotid artery for 14 days. Cystometry and bladder blood flow (BBF) assessment were performed 30 min after the last injection. After finishing cystometric investigations the rats were put in metabolic cages (3700M071, Tecniplast) for 24 h to assess urine production (UP), heart rate (HR), and MAP. Then the bladders of experimental rats were removed, fixed in buffered 10% formalin, and processed for paraffin blocks to conduct biochemical analyses. Finally, the animals were sacrificed by decapitation (the brains were stored for future studies). A detailed description of the animal model used in this study and of all experimental procedures was presented in a previous study [10].
All experimental procedures were performed in accordance with the European Communities Council Directive of 22 September 2010 (2010/63/EU) and were approved by the Local Ethics Committee (324/2018).
Surgical procedures
The applied surgical procedures have been described in detail in a previous study [10]. All surgical procedures were performed under general anaesthesia with intraperitoneal injection of 15 mg/kg of xylazine (Sedazin, Biowet, Puławy, Poland) and 75 mg/kg of ketamine hydrochloride (Ketanest, Pfizer, Warsaw, Poland). The abdominal wall was opened through an approximately 10 mm vertical midline incision. Then, a double lumen catheter was inserted through the apex of the bladder dome and fixed with 6–0 absorbable suture. In the same session a cannula was inserted into the carotid artery and filled with 40 IU/ml heparinised saline in order to ease drug administration directly to the bloodstream and to measure cardiovascular system parameters: HR and MAP.
Drugs
Nebivolol hydrochloride (NEBI): rel-(aR,a′R,2R,2′S) -a,a′-[iminobis(methylene)]bis[6-fluoro-3,4-dihydro-2H-1-benzopyran-2-methanol] hydrochloride) was dissolved in saline and administered intra – arterially via a polyethylene catheter inserted into the carotid artery in a single daily dose of 0.05 mg/kg. NEBI dosage was chosen according to literature data [8] and confirmed in a pilot study.
Cystometry and bladder blood flow
Conscious cystometry was performed via a bladder catheter connected to a microinjection pump (CMA 100) and to a pressure transducer (FT03, Grass Technologies). During the examination the bladder was filled with physiological saline at a constant rate of 0.05 ml/min. Micturition volumes were measured using a fluid collector attached to a force displacement transducer (FT03C, Grass Technologies). Cystometric profiles and micturition volumes were recorded continuously on a Grass polygraph and were determined graphically. The measurements in each rat represent average values of five bladder micturition cycles after obtaining repetitive voiding. Data on five reproducible micturition cycles for each animal were analysed, and a mean ± standard error of the mean (SEM) in each experimental group was calculated. The following cystometric parameters were assessed:
basal pressure (BP, cm H2O),
threshold pressure (TP, cm H2O),
micturition voiding pressure (MVP, cm H2O),
voided volume (VV, ml),
post – void residual (PVR, ml) – bladder capacity minus voided volume/fluid remaining in the bladder at the end of micturition,
volume threshold (VT, ml),
voiding efficiency (VE, %),
intercontraction interval (ICI, s),
bladder contraction duration (BCD, s),
relaxation time (RT, s),
bladder compliance (BC, ml/cm H2O) – calculated as the bladder capacity divided by the difference in the pressure threshold and baseline pressure using the formula [(VV + PVR)/(TP – BP)],
detrusor overactivity index (DOI, cm H2O/ml) – depicted as the quotient of the sum of amplitudes of all detrusor contractions during the filling phase and functional bladder capacity,
nonvoiding contraction amplitude (ANVC, cm H2O),
nonvoiding contraction frequency (FNVC, times/filling phase),
volume threshold to elicit NVC (VTNVC, %).
The BBF was assessed by laser Doppler blood perfusion imager (PeriScan PIM III, Perimed) and displayed as changes in frequency with a colour scale. BBF was measured five times for each rat’s bladder immediately after bladder emptying.
UP and cardiovascular parameters
After cystometry the rats were observed in metabolic cages (3700M071, Tecniplast, Buguggiate, Italy) for 24 h to investigate the effects of the examined substances on UP, HR, and MAP.
Biochemical analyses
Levels of the following biomarkers were measured in the urinary bladder tissue collected from the tested animals: interleukin 1β (IL-1β; ELISA Kit for IL1b, Cloud-Clone, Katy, TX), interleukin 6 (IL-6; Rat IL6 ELISA Kit, LifeSpan BioSciences, Seattle, WA), tumour necrosis factor α (TNF-α; ELISA Kit for TNF Alpha, LifeSpan BioSciences), nerve growth factor (NGF; Rat NGF ELISA Kit, LifeSpan BioSciences), and brain-derived neurotrophic factor (BDNF; Emax ImmunoAssay System, Promega, Madison, WI).
Results
Cystometry and bladder blood flow
Comparative analysis of WHY and SHR cystometrograms showed statistically significant differences. An increase in FNVC, ANVC, DOI, TP, and BP was observed, while a decrease in VTNVC, BC, ICI, VT, and VV was demonstrated. There were no statistically significant differences in BCD, RT, MVP, and PVRVE between these groups (Figures 1–3).
Figure 1
Cystometric parameters in the study groups. A – Basal pressure changes in different study groups: *WHY vs. SHR – p < 0.05, ^SHR vs. SHR + NEBI – p < 0.05. B – Threshold pressure changes in different study groups: **WHY vs. SHR – p < 0.01, ^SHR vs. SHR + NEBI – p < 0.05. C – Micturition voiding pressure changes in different study groups. D – Voided volume changes in different study groups: ***WHY vs. SHR – p < 0.001, ^^^SHR vs. SHR + NEBI – p < 0.001. E – Post-void residual changes in different study groups. F – Volume threshold changes in different study groups: **WHY vs. SHR – p < 0.01, ^^^SHR vs. SHR + NEBI – p < 0.001. All results are presented as the means ± SEM (n = 15 rats per group). The obtained data were assessed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. *Significantly different from the WHY group. ^Significantly different from the SHR group. * or ^ – p < 0.05; ** or ^^ – p < 0.01; *** or ^^^ – p < 0.001
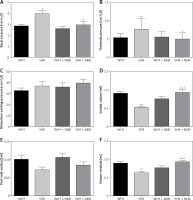
Figure 2
Cystometric parameters in the study groups. A – Voiding efficiency changes in different study groups. B – Intercontraction interval changes in different study groups: ***WHY vs. SHR – p < 0.001, ^SHR vs. SHR + NEBI – p < 0.05. C – Bladder contraction duration changes in different study groups. D – Relaxation time changes in different study groups. E – Bladder compliance changes in different study groups: **WHY vs. SHR – p < 0.01, ^SHR vs. SHR + NEBI – p < 0.05. F – Detrusor overactivity index changes in different study groups: ***WHY vs. SHR – p < 0.001, ^^^SHR vs. SHR + NEBI – p < 0.001. All results are presented as the means ± SEM (n = 15 rats per group). The obtained data were assessed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. *Significantly different from the WHY group. ^Significantly different from the SHR group. * or ^ – p < 0.05; ** or ^^ – p < 0.01; *** or ^^^ – p < 0.001
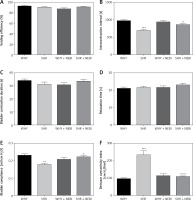
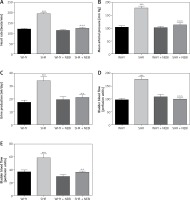
Figure 3
Cystometric parameters in the study groups. A – Amplitude of nonvoiding contraction changes in different study groups: ***WHY vs. SHR – p < 0.001, ^^^SHR vs. SHR + NEBI – p < 0.001. B – Frequency of non-voiding contractions changes in different study groups: ***WHY vs. SHR – p < 0.001, ^^^SHR vs. SHR + NEBI – p < 0.001. C – Changes of volume threshold to elicit non-voiding contractions in different study groups: ***WHY vs. SHR – p < 0.001, ^^SHR vs. SHR + NEBI – p < 0.001. All results are presented as the means ± SEM (n = 15 rats per group). The obtained data were assessed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. *Significantly different from the WHY group. ^Significantly different from the SHR group. * or ^ – p < 0.05; ** or ^^ – p < 0.01; *** or ^^^ – p < 0.001

The administration of NEBI in a 0.05 mg/kg/day dose did not cause any significant cystometric changes in WHY. On the contrary, in SHR NEBI normalised the changes in cystometric parameters characteristic for DO: the mean values of assessed parameters in SHR + NEBI were not significantly different in comparison to those in WHY. NEBI decreased FNVC, ANVC, DOI, TP, and BP and simultaneously increased VTNVC, BC, ICI, VT, and VV to similar values as those observed in WHY. No significant differences in BCD, RT, MVP, PVR, and VE were observed between any of the studied groups (Figures 1–3).
The basic BBF level was lower in SHR than in WHY. The administration of NEBI had no influence on BBF in WHY, but significantly increased BBF in SHR (Figure 4 D).
Figure 4
The measurements of heart rate, mean arterial pressure, urine production, and bladder blood flow parameters in the study groups. A – Heart rate changes in different study groups: *WHY vs. SHR – p < 0.05, ^^SHR vs. SHR + NEBI – p < 0.01. B – Mean arterial pressure changes in different study groups: ***WHY vs. SHR – p < 0.001, ^^^SHR vs. SHR + NEBI – p < 0.001. C – Urine production changes in different study groups. D, E – Bladder blood flow changes in different study groups: ***WHY vs. SHR – p < 0.001, ^SHR vs. SHR + NEBI – p < 0.05. All results are presented as the means ± SEM (n = 15 rats per group). The obtained data were assessed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. *Significantly different from the WHY group. ^Significantly different from the SHR group. * or ^ – p < 0.05; ** or ^^ – p < 0.01; *** or ^^^ – p < 0.001
UP and cardiovascular parameters
Mean values of HR were lower in SHR in comparison to WHY. The administration of NEBI in WHY had no statistically significant impact on HR, while in SHR it triggered an increase up to the values observed in the WHY group (Figure 4 A). Higher values of MAP were observed in SHR in comparison to WHY. The administration of NEBI led to the decrease in MAP in SHR, while MAP in WHY remained unchanged (Figure 4 B). There were no statistically significant differences in UP between the experimental groups (Figure 4 C).
Biochemical analysis
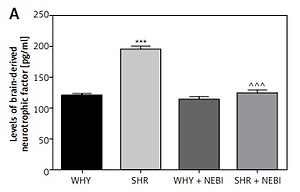
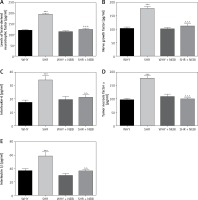
The analysis of biomarkers characteristic for DO revealed statistically significant differences. The levels of BDNF and NGF, as well as pro-inflammatory markers (IL-6, TNF-α, and IL-1β), were elevated in SHR compared to WHY. Administration of NEBI significantly decreased the levels of all these biomarkers in SHR, while it had no influence on WHY (Figure 5).
Figure 5
Biochemical parameters in the study groups. A – Brain-derived neurotrophic factor changes in different study groups: ***WHY vs. SHR – p < 0.001, ^^^SHR vs. SHR + NEBI – p < 0.001. B – Nerve growth factor changes in different study groups: ***WHY vs. SHR – p < 0.001, ^^^SHR vs. SHR + NEBI – p < 0.001. C – Interleukin-6 changes in different study groups: ***WHY vs. SHR – p < 0.001, ^^SHR vs. SHR + NEBI – p < 0.01. D – Tumour necrosis factor α changes in different study groups: ***WHY vs. SHR – p < 0.001, ^^^SHR vs. SHR + NEBI – p < 0.001. E – Interleukin-1β changes in different study groups: ***WHY vs. SHR – p < 0.001, ^^SHR vs. SHR + NEBI – p < 0.01. All results are presented as the means ± SEM (n = 15 rats per group). The obtained data were assessed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. *Significantly different from the WHY group. ^Significantly different from the SHR group. * or ^ – p < 0.05; ** or ^^ – p < 0.01; *** or ^^^ – p < 0.001
Discussion
The main finding of this study is that the treatment with NEBI resulted in an improvement of cystometric parameters characteristic for OAB in SHR. The decrease in DOI is particularly important because DOI quantifies detrusor overactivity, and the measurement of this parameter is widely used in urodynamic testing in human patients. Moreover, we have found that it normalises MAP in SHR.
The results obtained in this study are in concordance with data described in the current literature. The alterations in cystometrograms in SHR treated with NEBI were significant. It has been reported that the usage of β3-AR agonists ameliorates DO and reduces the number of frequency and urgency episodes [11]. Stimulation of β3-AR induced the increment in BC and VV values, with no impact on MVP or PVR. After treatment with NEBI a reduction in DOI, which characterises the contractile activity of the detrusor muscle cells and correlates with severity of DO, was observed [12]. The raise in VTNVC after administration of NEBI correlated with the increase in ICI, along with the decrease in FNVC, indicating the effectiveness in DO reduction. This effect suggests an influence of this drug on afferent mechanisms that regulate the micturition cycle. Because NEBI administration did not change MVP, PVR, VE, BCD, or RT in SHR it seems that this drug does not impair urine storage.
NEBI, a β1-AR antagonist with β3-AR agonistic activity, was chosen for this study due to its potential relaxing effect on the bladder, but also due to the fact that it is approved for hypertension treatment. Our findings indicate that NEBI may not only alleviate detrusor overactivity but also normalise MAP in SHR. In WHY the cardiovascular parameters remained unchanged. NEBI used in SHR improved BBF and did not affect UP. The dose of the drug administered in this study did not change the micturition frequency or BBF in WHY. A decrease in BBF in untreated SHRs was also previously reported by Yono et al. [13].
Interestingly, NGF and BDNF are possible markers of OAB [14]. It has been reported that urinary protein levels, such as NGF and BDNF, increase in patients with OAB, bladder outlet obstruction, and DO [15]. The urinary NGF level increases physiologically in normal subjects at urge to void but increases pathologically in OAB patients at small bladder volume and with a sensation of urgency. However, BDNF sequestration does not affect the physiological voiding reflex, which proves that BDNF modulates bladder activity only in the case of its malfunctioning. In our study we found that both NGF and BDNF levels were increased in SHRs, and both were normalised by the administration of NEBI. Therefore, it seems that NGF and BDNF may become more than just useful markers for OAB diagnosis, but also prove applicability for assessing the effectiveness of pharmacological treatment. Similarly, the levels of pro-inflammatory markers (IL-6, TNF-α, and IL-1β) were increased in SHRs and normalised by administration of NEBI.
Our results may have clinical implications for the treatment of OAB, especially in the case of concomitant hypertension. The prevalence of OAB is greater among the elderly, and the highest percentage of concomitant diseases includes cardiovascular comorbidities, especially hypertension [16]. Hypertension has become the most prevalent chronic disease worldwide [17]. Nocturnal polyuria and essential hypertension are manifestations of the same pathophysiological process, in which resetting of the pressure-natriuresis relation in the kidney leads to sodium retention and elevated blood pressure [18]. McKeigue and Reynard propose the age-related impairment of endothelial NO release as a possible mechanism for sodium retention [18]. Elevated HR and hypertension are correlated with nocturia via sympathetic overactivity. NEBI, due to its NO potentiating and vasodilatory effect, may reduce those symptoms. Currently used medications in the treatment of OAB may have a negative influence on the cardiovascular system. Antimuscarinics induce changes in blood pressure, pulse rate, or ECG, particularly QT prolongation and induction of polymorphic ventricular tachycardia, and increases in HR [19]. In the case of mirabegron, even if the cardiovascular effects of mirabegron observed in clinical studies have been minimal, effects on the heart rate and blood pressure need to be monitored when the drug is prescribed [20]. Mirabegron treatment is contraindicated in patients with severe uncontrolled hypertension. Regular monitoring of blood pressure is important during the treatment, especially in patients with pre-existing hypertension [5]. In our study NEBI did not affect the studied cardiovascular parameters in WHY and, more importantly, normalised MAP in SHR. It seems to be a promising drug in the treatment of OAB patients with pre-existing hypertension.
In conclusion, NEBI alleviates OAB symptoms and normalises blood pressure in SHRs. These results suggest that NEBI may be a useful treatment alternative for OAB patients with pre-existing hypertension; this hypothesis should be verified in future clinical trials.