Colorectal cancer (CRC) poses a major global health burden. It is the third most commonly diagnosed cancer (≈10% of all cases) and the second leading cause of cancer-related mortality worldwide (≈9.2% of all deaths) [1]. With an estimated 1.93 million new diagnoses annually, 20–30% of cases present with metastatic disease at diagnosis. A frequent and serious complication in CRC management is deep vein thrombosis (DVT), which constitutes over 68% of all venous thromboembolism (VTE) events [2]. CRC patients face a 4.7-fold higher risk of developing DVT compared to the general population [3]. DVT, anastomotic leak, ileus, and surgical site infection (SSI) are frequently observed in patients following colorectal cancer surgery, often leading to rapid clinical deterioration and even mortality. CRC promotes hypercoagulability and thrombosis risk throughout its progression. Early-stage tumors (I–II) initiate a procoagulant state via tissue factor overexpression and microparticle release. With nodal involvement (stage III), increased tumor burden and systemic inflammation (e.g., IL-6, TNF-α) amplify coagulation, while surgery and chemotherapy further elevate thrombosis risk. In metastatic disease (stage IV), extensive tumor load, liver dysfunction, vascular obstruction by lung metastases, and cachexia-induced stasis culminate in the highest risk of life-threatening thrombosis, necessitating mandatory thromboprophylaxis.
The classic presentation of deep vein thrombosis (DVT) includes sudden-onset swelling in one limb, localized tenderness, and changes in skin coloration. Key nursing interventions consist of instructing the patient to maintain strict bed rest and immobilize the affected limb, utilizing physical prophylaxis such as graduated compression stockings (GCS) or intermittent pneumatic compression (IPC) devices, and administering prescribed anticoagulant medications such as low-molecular-weight heparin (LMWH) [4, 5]. Current prophylactic strategies for DVT in CRC patients remain insufficient, with fewer than 25% receiving guideline-adherent care [6]. This challenge is compounded by the limited specificity of available risk assessment tools and conventional biomarkers. In this context, the present study aimed to investigate the diagnostic and therapeutic potential of TCN1 – a key vitamin B12 transporter – in CRC-associated DVT, with particular focus on its role within the TGF-β/SMAD2 signaling pathway and the therapeutic opportunities arising from modulation of the vitamin B12 pathway.
Methods
Measurement of TCN1 mRNA levels in colorectal cancer patients
A retrospective case-control study was conducted using the clinical database of Nantong Second People’s Hospital. The study population was identified from all consecutive CRC patients treated between May 2022 and June 2023. Cases were defined as CRC patients with imaging-confirmed DVT. Controls were selected from CRC patients without DVT during the same period, matched for age (±5 years), sex, and tumor stage. Eligibility required histologically confirmed CRC. Key exclusion criteria comprised recent anticoagulant use (within 14 days prior to blood draw or DVT diagnosis), inherited thrombophilia, or active infection, to minimize confounding factors. Blood samples for TCN1 measurement were obtained prior to any therapeutic intervention for DVT. Medication records were reviewed to identify patients who received hydroxocobalamin during their clinical course.
Peripheral blood samples were processed within 30 min of collection through centrifugation at 3,000 g for 15 min at 4°C. RNA samples meeting quality criteria (A260/A280 > 1.8) were subjected to TCN1 mRNA quantification using SYBR Green-based RT-PCR (Applied Biosystems). The study protocol was approved by the institutional ethics committee (Approval No: 2022-085).
Detection of TCN1 expression by immunofluorescence
Lovo cells, authenticated by STR profiling, were cultured in RPMI-1640 medium supplemented with 10% FBS. For functional studies, cells were transfected with TCN1-specific primers using Lipofectamine 3000 reagent at a DNA-to-reagent ratio of 2.5 µg : 5 µl. Subsequently, TCN1 expression was assessed by immunofluorescence using an anti-TCN1 primary antibody (1 : 500, 4°C) followed by a fluorescently conjugated secondary antibody (1 : 200) and DAPI counterstaining. All samples were imaged with a NIKON Ts2R-FL microscope, with a minimum of three replicates per experimental condition.
Assessment of TCN1-KD in thrombus-bearing colon cancer mice
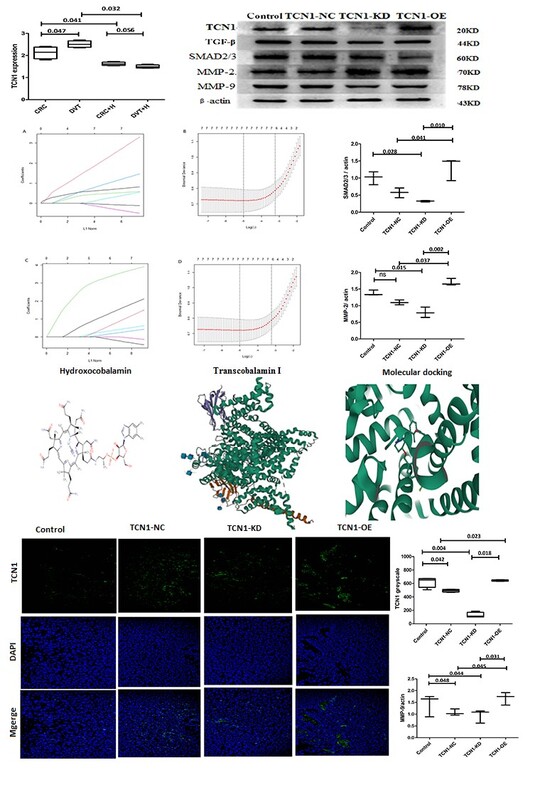
Forty SPF BALB/c-nu mice (Certification: SCXK 20240007; Ethics Approval: 23083) were subcutaneously injected with Lovo cells (7 × 106 cells in 0.2 ml of PBS). Upon reaching a tumor volume of ~0.2 cm³, mice were randomized to four groups (n = 10): PBS control, TCN1-NC (non-targeting siRNA + hydroxocobalamin), TCN1-KD (knockdown + hydroxocobalamin), and TCN1-OE (overexpression + hydroxocobalamin). Hydroxocobalamin (2 mg/kg) was administered intraperitoneally daily from day 7. Tumor volume was calculated as 0.5 × L × W², and thrombus weight was measured at endpoint.
A separate sodium laurate-induced venous injury model was established in BALB/c mice. Under aseptic conditions and 4% isoflurane anesthesia, the femoral vein was exposed via blunt dissection, occluded with a vascular clamp, and injected with sodium laurate (4 mg/kg; sham controls received 0.9% NaCl). After 20 s of occlusion, the clamp was released. Postoperative procedures included wound closure and penicillin G administration (40,000 U, i.m.). Serum was collected 24 h after surgery via lower limb venipuncture and centrifuged at 3,500 rpm (16 cm radius, 15 min).
Diagnostic evaluation and drug-gene interaction
Sodium laurate-induced venous thrombus was assessed by quantifying vWF and TXB2 levels via ELISA. Western blotting was used to examine protein expression of TGF-β, SMAD2/3, MMP-2, and MMP-9 (antibodies from Affinity and Abcam; ACTB as loading control). Colorectal adenocarcinoma (COADREAD, GSE41258) and deep venous thrombosis (DVT, GSE19151) datasets were retrieved from the NCBI GEO database. Drug-gene interactions were predicted using the DrugBank database and visualized with Cytoscape (v3.8.2). Molecular docking simulations were conducted with AutoDock Vina to evaluate binding affinity between hydroxocobalamin and TCN1 (affinity < –7.0 kcal/mol). Diagnostic biomarkers were selected using LASSO regression (λ = 0.01, AUC > 0.7).
Statistical analysis
Statistical analyses were performed using ANCOVA, with age and tumor stage included as covariates. TGF-β, SMAD2/3, MMP-2, and MMP-9 were expressed as mean ± standard deviation (SD), and were compared using Student’s t-test. Two-tailed p-values of < 0.05 were considered statistically significant.
Results
TCN1 expression was significantly elevated in CRC patients with DVT (n = 275) compared to matched non-DVT controls (n = 275) (p < 0.05), showing a 4.2-fold increase (p < 0.01). This upregulation was most pronounced in younger patients (21–40 years) and declined gradually with age, though it remained elevated across all groups (41–60 and 61–80 years; p < 0.05). TCN1 expression was markedly higher across DVT subtypes relative to controls (p < 0.01), with DVT patients exhibiting a 4.2-fold increase in TCN1 expression (p < 0.01). Hydroxocobalamin intervention significantly reduced TCN1 expression in DVT patients (p < 0.01).
Successful transfection and modulation of TCN1 expression in CRC cells were confirmed by immunofluorescence and Western blot. TCN1 knockdown (KD) significantly suppressed the protein levels of TGF-β and phosphorylated SMAD2/3, while TCN1 overexpression (OE) enhanced their activation (p < 0.01). Consistent with pathway inhibition, TCN1-KD also led to downregulation of MMP-2 and MMP-9 (p < 0.01), suggesting a role in vascular remodeling. Furthermore, hydroxocobalamin treatment markedly reduced serum levels of the thrombosis markers TXB2 and vWF by 53% (p < 0.01). This suppression was reversed under TCN1-KD conditions (p < 0.01), supporting the involvement of TCN1 in the regulation of endothelial dysfunction and platelet aggregation.
Transcriptomic analysis identified 2,541 upregulated and 2,146 downregulated genes in CRC, and 1,432 upregulated and 1,105 downregulated genes in DVT. GO enrichment analysis highlighted TCN1-related pathways including cobalt ion transport, cobalamin binding, and TGF-β receptor activity. LASSO regression retained all seven genes as diagnostic candidates for CRC and DVT. Clinical validation confirmed significant expression differences for six genes in CRC (PLA2G16, THOC2, SMAD5, TCN1, KIAA1324, NLRP2; p < 0.05) and four genes in DVT (PLA2G16, THOC2; p < 0.05). Three consistently upregulated genes – PLA2G16, THOC2, and TCN1 – were selected as key biomarkers. ROC analysis demonstrated THOC2 (0.72, 95% CI: 0.61–0.83) with AUC > 0.7 in both training and validation sets, while PLA2G16 (0.71, 95% CI: 0.64–0.79) and TCN1 (0.63, 95% CI: 0.48–0.78) each achieved AUC > 0.6. TCN1 was the only gene with predicted small molecule drug interactions, identifying hydroxocobalamin and cyanocobalamin as associated therapeutic agents.
Discussion
This study establishes TCN1 as a novel mediator in CRC-associated DVT through its involvement in TGF-β/SMAD2/3 signaling. The elevation of TCN1 in DVT patients exceeds the diagnostic sensitivity of conventional biomarkers such as D-dimer [7–14]. We observed an inverse relationship between TCN1 expression and age, with peak levels in younger patients and a decline in older age group, consistent with known age-related increases in thrombosis risk among CRC populations. Functional studies demonstrated that TCN1 overexpression enhances SMAD2/3 phosphorylation and upregulates MMP-2 and MMP-9, while knockdown suppresses this pathway. Hydroxocobalamin significantly reduced thrombotic markers (vWF and TXB2), consistent with its high binding affinity for TCN1 and supporting its potential for clinical repurposing, given its established safety profile.
These findings on TCN1 and vitamin B12 pathway modulation can be contextualized within the growing body of research on nutrient-sensing receptors in CRC. For instance, recent studies have identified vitexin as a novel vitamin D receptor (VDR) agonist that mitigates the transition from chronic intestinal inflammation to colorectal cancer [15]. Similar to VDR activation – which exerts anti-inflammatory, anti-proliferative, and pro-differentiation effects – targeting TCN1 with hydroxocobalamin may modulate the tumor microenvironment and thrombotic complications, suggesting a broader theme of nutrient-related pathways influencing CRC progression and associated complications.
The TCN1-PLA2G16-THOC2 biomarker signature demonstrated superior diagnostic performance compared to current standards, with THOC2 achieving an AUC exceeding 0.7 compared to fibrinogen (AUC ~0.6). TCN1’s dual role as both a druggable target and a biomarker offers distinctive clinical utility. PLA2G16 may link phospholipase activity to platelet aggregation, while hydroxocobalamin enables targeted prophylactic intervention.
Several limitations should be acknowledged, including the retrospective study design and the inherent constraints of xenograft models in fully recapitulating human thrombosis. Future efforts should focus on multi-center validation, development of standardized TCN1 assays, and well-controlled interventional trials.