Myelofibrosis (MF) is a type of Ph-negative myeloproliferative neoplasm that may be primary or secondary, evolving from polycythemia vera (PPV-MF) or essential thrombocythemia (PET-MF). Prognosis varies, with overall survival (OS) ranging from 1 to 15 years. Prognostic tools such as DIPSS, DIPSS-plus, MIPSSv2.0, and GIPSS incorporate clinical, cytogenetic, and molecular data. Allogeneic stem cell transplantation (HSCT) is the only curative option, but many patients are ineligible due to age or comorbidities. Treatments include danazol, recombinant erythropoietin, thalidomide, and momelotinib for anemia, and JAK1/2 inhibitors such as ruxolitinib and fedratinib for constitutional symptoms and splenomegaly [1–4].
Ruxolitinib (Rux) is the first Janus kinase (JAK1/JAK2) inhibitor to be approved by the US Food and Drug Administration (FDA), in 2011, for the treatment of intermediate or high-risk patients with all forms of MF. Rux demonstrated a significant effect in reducing constitutional symptoms and improving quality of life in 29% and 42% of patients in the COMFORT-1 and COMFORT-2 studies, respectively [5, 6].
In this retrospective study, we aimed to document real-life clinical experiences with Rux in MF patients. We evaluated the impact of DIPSS-plus scores, splenic response, baseline peripheral blood platelet and blast counts, and initial Rux dose on overall patient survival, including progression-free survival (PFS).
Methods
This analysis includes 54 MF patients (26 men, 48.1%) treated with Rux. Treatment was primarily for symptomatic splenomegaly and constitutional symptoms. Twenty-eight patients had primary MF, and 26 had secondary MF (21 PPV-MF, 5 PET-MF). Median age was 65 years at diagnosis, 68 years at Rux initiation. Median follow-up was 69.5 months (M) from diagnosis, 29 M from Rux initiation. Median palpable spleen size was 15 cm. Cytogenetic results were available for 49 (90.7%) patients at baseline: 36 (66.7%) patients had a normal karyotype, 5 (9.3%) patients had trisomy 8, 1 (1.8%) patient had a deletion of chromosome 7, 1 (1.8%) patient had a deletion of chromosome 20, and other abnormalities were observed in 6 cases.
At the start of treatment, 17 (31.5%) patients had a hemoglobin value < 100 g/l in their blood counts, of which 9 (16.7%) were transfusion dependent. Platelet values ≤ 150.109/l were present in 16 (29.6%) patients and peripheral blood blasts ≥ 2% in 13 (24.1%) patients (Table I).
Table I
Patient demographics and baseline clinical characteristics of 54 patients
| Parameter | Value |
|---|---|
| Male sex; N (%) | 26 (48.1) |
| Median age at MF diagnosis [years] (range) | 65 (41–80) |
| Median age at Rux initiation [years] (range) | 68 (46–80) |
| MF type, N (%) | |
| Primary MF | 28 (51.9) |
| PPV-MF | 21 (38.8) |
| PET-MF | 5 (9.3) |
| Mutational status; N (%) | |
| JAK2V617F mutated | 43 (79.6) |
| CALR mutated | 2 (3.7) |
| Triple negative | 9 (16.7) |
| DIPSS-plus risk category; N (%) | |
| Intermediate-1 | |
| Intermediate-2 | |
| High | |
| Hemoglobin < 100g/l; N (%) Transfusion dependency; N (%) | 17 (31.5) 9 (16.7) |
| Platelets ≤ 150.109/l; N (%) | 16 (29.6) |
| Peripheral blood blasts ≥ 2%; N (%) | 13 (24.1) |
| Unfavorable karyotype; N (%)# | 8/49 (14.8) |
| Splenomegaly at Rux initiation; N (%) | |
| Mild splenomegaly < 10 cm | 9 (16.7) |
| Moderate splenomegaly 10–20 cm | 41 (76.0) |
| Severe splenomegaly > 20 cm | 4 (7.3) |
| Median TSS; range | 82 (72–95) |
| Median time from diagnosis to Rux [months] (range) | 24 (3–96) |
| Prior therapy*; N (%) | |
| Hydroxyurea | 39 (72.2) |
| Hydroxyurea and anagrelide | 9 (16.6) |
| Spleen radiotherapy | 2 (3.7) |
| Hydroxyurea, anagrelide and HSCT | 1 (1.9) |
| None | 3 (5.6) |
| Starting Rux doses; N (%) | |
| 10 mg | 2 (3.7) |
| 20 mg | 21 (38.8) |
| 30 mg | 19 (35.3) |
| 40 mg | 12 (22.2) |
Median time from diagnosis to Rux initiation was 24 M; median treatment duration was 18 M. Based on baseline platelet values, patients started Rux treatment at a dose of 10–40 mg/dose/daily, with a median daily dose of 30 mg. During treatment, due to toxicity, the dose of Rux had to be reduced in 22 (40.7%) patients; in 5 (9.3%) patients, when platelet values improved, the dose could be increased; 28 (51.9%) patients continued the same dose.
Evaluation of treatment outcomes, statistical analysis
Splenomegaly was measured by palpation with determination of overlap of the amount of splenic size over the rib arch, and patients were divided into three groups according to the splenic response achieved at 6 M: major splenic response (splenomegaly regression > 75% from baseline), mild response (splenomegaly regression by 35–75%) and patients with minimal/no response (splenomegaly regression < 35%). The evolution of constitutional symptoms was evaluated using the MPN-SAF TSS questionnaire (Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score). The toxicity of Rux treatment was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [7, 8].
Results
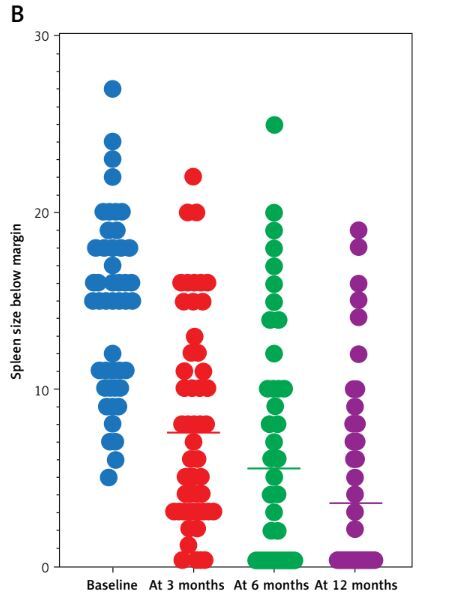
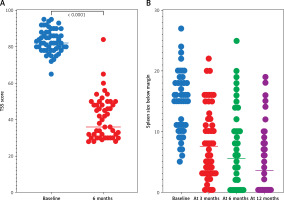
Constitutional symptoms improved in 52 (96.3%) patients within 6 weeks. Median TSS dropped from 82 to 36 at 6 M; 26 patients had ≥ 50% improvement (p < 0.0001). Median spleen size dropped from 15 cm to 6 cm at 6 M, and to 2.5 cm at 12 M. Spleen response was present in a total of 41 (75.9%) patients: 22 (40.7%) major, 19 (35.2%) mild, 13 (24.1%) minimal/no (p < 0.0001). Thirteen (24.1%) patients had minimal/no spleen reduction, classified as primary resistance. Secondary resistance to Rux treatment developed in 7 (13%) patients after 20–52 M (Figures 1 A, B).
Figure 1
A – Effects of ruxolitinib treatment on TSS (baseline median TSS was 82 (range: 72–95), median TSS at month 6 was 36 (range: 25–90; p < 0.0001). B – Effects of ruxolitinib treatment on spleen response (baseline median size of the spleen below the rib arch was 15 cm (range: 5–27 cm), median spleen size at month 6 was 6 cm (range: 0–25 cm) and at month 12 of treatment it was 2.5 cm (range: 0–17 cm; p < 0.0001)
Anemia worsened in 19 (35.2%) patients, neutropenia in 4 (7.4%), thrombocytopenia in 12 (22.2%). Three patients became transfusion-independent. Two patients discontinued Rux due to thrombocytopenia grade IV. Non-hematologic toxicities were mostly mild. Pneumonia developed in 3 (5.6%) patients, and in 2 cases pneumonia led to hospitalization. Among other adverse events, the following occurred: fatigue (N = 6; 11.1%), diarrhea (N = 5; 9.3%), headache (N = 3; 5.6%), musculoskeletal pain (N = 2; 3.7%). Elevation of liver function test values occurred in 2 (3.7%) cases.
In 17/20 (85.0%) cases, deaths were related to underlying disease or progression to acute leukemia; only 3/20 (15.0%) cases involved other causes of death (cardiac cause and cerebrovascular hemorrhage). Nineteen deaths occurred after stopping Rux treatment, while only one death (cardiovascular cause) occurred during treatment. After Rux treatment failure, treatment included HSCT (N = 7; 13%), fedratinib (N = 3; 5.6%), splenic radiation (N = 2; 3.7%), clinical trials (N = 1; 1.9%), azacitidine + venetoclax (N = 1; 1.9%), or supportive care (N = 9; 16.7%).
The expected 4-year OS in patients with primary or secondary MF did not differ (63% vs. 75%; p = 0.620, respectively), nor did 4-year PFS differ by type of MF (16% vs. 41%; p = 0.168, respectively). However, significantly different OS was demonstrated by the baseline DIPSS-plus score: 96% vs. 85% vs. 0% in the intermediate-1, intermediate-2, and high-risk groups (p < 0.0001). The impact of DIPSS-plus score on PFS was also confirmed (p < 0.0001).
We confirmed the advantage of achieving at least a mild splenic response in terms of expected OS, with OS rates of 42%, 93%, and 93% (p = 0.006) according to no/minimal response vs. mild vs. major response, respectively. A clear advantage of achieving at least a mild splenic response on PFS was also found (0% vs. 64%; p < 0.0001).
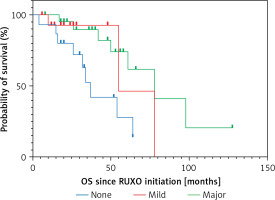
We evaluated the impact of the initial daily dose of Rux (10–20 mg vs. 30–40 mg/daily), peripheral blood blasts (< 2% vs. ≥ 2%) and platelet count at treatment initiation (≤ 150.109/l vs. > 150.109/l) on patient survival. We found a significant difference between the groups, with the following 4-year OS: the initial dose of Rux 48% vs. 85% (p = 0.014), the blast value 50% vs. 81% (p = 0. 030), and according to the platelet value 50% vs. 76% (p = 0.008) (Figure 2).
Discussion
Our analysis demonstrated a significant effect of Rux treatment on constitutional symptoms and the improvement of overall quality of life in 52 (96.3%) patients. The effect of Rux on spleen size reduction was also significant, as a reduction of spleen size by palpation at 6 M of treatment of > 35% was present in 41 patients (75.9%), which is a larger treatment effect compared to the results of the COMFORT-I and II studies, but comparable to the results of the MYNERVA project (42%, 32%, and 66%, respectively) [5, 6, 9].
During Rux treatment, anemia, neutropenia, and thrombocytopenia of all grades occurred in 19 (35.2%), 4 (7.4%), and 12 (22.2%) patients, respectively. This lower incidence compared to the JUMP study (anemia 56.3%; thrombocytopenia 42.2%) is probably due to our more significant reduction in the initial dose of Rux in some cases [10]. We discontinued treatment due to the development of grade 4 thrombocytopenia in only two cases (3.7%). We found that Rux is well tolerated by patients and the nonhematological toxicity present was mostly grade 1/2. According to published data, the most common event was the occurrence of infections, in 9 patients (16.4%), but only 2 (3.6%) cases were grade 4. A meta-analysis of clinical trials, including COMFORT trials, confirmed that Rux treatment does not significantly increase the risk of infections in patients with MF, and our analysis confirmed similar findings. To date, no type of secondary malignancy, including skin malignancy, has been demonstrated in our cohort [11, 12].
Our analysis confirmed the already published conclusions that achieving any splenic response correlates with a longer overall survival in patients (93% and 93% vs. 42%, respectively) (p = 0.006) and correlates highly significantly with the risk of disease progression (p < 0.0001) [13]. It is a known fact that the splenic response and thus OS are dependent on the initial dose of Rux and our results are consistent with this observation: in the group with a starting dose of 10–20 mg daily, survival was 48% versus 85% in the group treated with 30–40 mg daily (p = 0.014).
Primary resistance to Rux therapy is an important therapeutic problem in MF. In our cohort, primary resistance was observed in 13 (24.1%) patients and, consistent with the results of Maffioli’s study, the splenic response can identify patients at risk as early as month 6 of treatment. These patients represent the highest risk group for disease progression and shortest survival [14]. In these cases, we should early consider other treatment options based on the patient’s age and comorbidities, including HSCT, fedratinib, momelotinib, or enrolling the patient in a clinical trial [15].
In conclusion, we found significantly better survival and a low incidence of inadequate response to Rux treatment in patients in the intermediate-1 DIPSS-plus risk group; however, this observation is likely attributable to the underlying biological behavior of the disease, which may render it more responsive to JAK inhibition.