With the evolution of the SARS-CoV-2 virus, multiple variants, such as Alpha, Beta, Lambda, Delta and Omicron, have been identified to date, causing challenges in preventing and controlling the pandemic [1, 2]. In particular, the Delta (B.1.617.2) variant found in India in 2021 has quickly become the common virus strain of the local outbreak in China. However, clinical data are lacking on the clinical characteristics, effective therapy strategies, and vaccine response of patients harbouring the Delta variant, presenting serious challenges to its treatment; meanwhile, people may be tired of adhering to preventive behaviours in the COVID-19 pandemic; thus, we aimed to investigate the vaccine effects and clinical characteristic for different variants [3].
Recently, the transmission and infection of the Delta variant via flights from India have rapidly caused regional outbreaks in the northwest cities of China. China has adopted an active isolation and therapy policy, and it is possible to observe the clinical characteristics, treatment effects, and vaccine responses of the Delta and wild-type SARS-CoV-2 strains.
Methods
Between 8 February 2020 and 10 January 2022, 44 patients with wild-type SARS-CoV-2 pneumonia (wild-type group) and 106 patients with Delta-variant pneumonia (Delta group) underwent isolated therapy by the treatment team of the First Affiliated Hospital of Xi’an Jiaotong University and Xi’an 8th Hospital. All the patients underwent nasopharyngeal swab sample collection according to the guidelines of the National Health Commission of the People’s Republic of China on Severe Acute Respiratory Syndrome Coronavirus Type 2 and were diagnosed via reverse transcription polymerase chain reaction of SARS-CoV-2 viral titre (Trial version 8) [4]. The results were confirmed by the Xi’an Centres for Disease Control and Prevention. This study was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University, and all the patients provided informed consent.
The baseline and clinical data of the two groups, including demographics, clinical features, laboratory results, chest CT and SARS-CoV-2 viral nucleic acid tests, were collected from the electronic medical record system. The incubation period was defined as the number of days from contact exposure to onset of the positive nucleic acid result. The vaccination information of the Delta variant patients was collected, and the values of IgG and IgM antibodies were detected by laboratory tests. All the data were extracted and analysed by 2 independent groups of clinicians (infectious disease and respiratory physician), and any disputed data were resolved through consultation with a third independent physician.
According to the definition of the National Health Commission of China, the clinical classification of SARS-CoV-2 included 4 types: mild, common, severe, and critical. The mild and common types were defined as non-severe, while severe and critical types were defined as severe [4]. After the patient’s symptoms disappeared and they had undergone a repeated swab test for SARS-CoV-2 nucleic acid, and 2 consecutive nucleic acid tests on throat swabs were negative (at least 24 h between the 2 tests), the patient was defined as negative for SARS-CoV-2. The time from the first positive to the negative results was defined as the primary virus clearance time.
Propensity score matching (PSM) was performed to adjust for significant differences in the baseline data and to reduce the impact of potential bias factors. Age, body weight, and adult sex were selected to calculate propensity scores, and p < 0.05 was considered significant. Categorical data were analysed using the χ2 test or analysis of variance, and continuous data were tested using paired and unpaired t tests. The protective effect of the SARS-CoV-2 vaccine was analysed by χ2 test, and odds ratio (OR) values and 95% confidence intervals (95% CI) were calculated.
Results
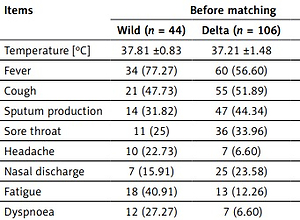
The Delta-variant group showed a higher infection rate in minors (14.16% vs. 0%, p = 0.02). The clinical and vaccine data are shown in Table I. Patients in the Delta variant group had higher vaccination rates (p < 0.001), and the incidence of anosmia or dysgeusia was higher than that in the wild-type group (p < 0.05), and these clinical features persisted after matching. Additionally, the Delta-variant group showed a lower incidence of severe classification than the wild-type group (p < 0.05). Unvaccinated patients had a 15.59-fold higher risk of severe classification than the vaccinated patients (OR = 15.59; 95% CI: 2.41–100.92; p < 0.001); after matching, the OR was 10 (95% CI: 2.09–46.77; p < 0.001).
Table I
Clinical features of patients with SARS-CoV-2 wild and Delta variant
The Delta variant group had a higher contact history and clustering disease features than the wild-type group (Table II, p < 0.05). Thus, a shorter incubation period was confirmed in the Delta variant group (p < 0.01). Although it showed high transmissible features, lung CT performance was similar between the Delta-variant and wild-type groups after matching, and the Ct values of the SARS-CoV-2 nucleic acid test were slightly higher than those of the wild-type group (p < 0.05). The IgG and IgM antibody titres of the vaccinated patients are shown in Table II. Blood biochemical test parameters indicated lower neutrophil and lymphocyte depletion in the Delta variant group (p < 0.01), and the C reactive protein (CRP) and erythrocyte sedimentation rate (ESR) values were significantly lower than those in the wild-type group (p < 0.05).
Table II
The auxiliary examination results of patients with SARS-CoV-2 wild and Delta variant
[i] Data are presented as n (%) or mean ± standard deviation. CT – computed tomography, WBC – white blood cell, NEUT – neutrophils, LYMP – lymphocytes, ALT – alanine aminotransferase, AST – aspartate aminotransferase, CRP – C-reactive protein, ESR – erythrocyte sedimentation rate. P-value, compared with the Delta-variant group.
Lower antiviral (52.83% vs. 93.18%), antibiotic (18.87% vs. 75%), and immunotherapy (5.66% vs. 68.18%) use and higher anticoagulation therapy (57.55% vs. 13.64%) use were observed in the Delta variant group (p < 0.01). All patients recovered after isolation treatment; however, the incidence of acute respiratory distress syndrome (ARDS) only occurred in the wild-type group (before matching: 9.09%, p = 0.01; after matching: 10%, p = 0.06). No myocardial infarction, stroke, or deep vein thrombosis occurred. The viral clearance time of SARS-CoV-2 was significantly faster in the Delta-variant group than in the wild-type group (before matching: 10.54 ±6.81 d vs. 18.62 ±12.41 d; after matching: 10.58 ±1.82 d vs. 14.87 ±4.62 d, p < 0.001).
Discussion
Previous studies have confirmed that the Delta variant has a faster spread and stronger transmission characteristic than earlier variants, possibly leading to increased hospitalizations and intensive care unit admission [5]. Our findings suggest that the patients in the Delta-variant group had a higher contact history with infected patients. Additionally, patients with the Delta variant showed more obvious characteristics of clustered disease. A more in-depth study found that the Delta variant has higher transmission efficiency and faster cell infectivity and even caused an infection rate of more than 90% in partial populations [6, 7]. These studies confirmed that the Delta-variant virus has stronger transmission and a higher incidence. Our study also confirmed that the Delta variant has a significantly shorter incubation period, even in the vaccinated population, showing this high transmission characteristic.
Early studies have shown that sudden loss of smell and taste may be early signs of SARS-CoV-2 infection in healthy adults and children [8, 9]. Our study confirmed that the incidence of taste and smell disorders in patients with the Delta variant was higher than that in the wild-type group. Additionally, systemic symptoms such as fatigue and headache were significantly higher in patients with the Delta variant, further suggesting that the Delta variant had higher transmissibility and virulence. The Delta variant showed higher consumption of lymphocytes and neutrophils, but the inflammatory index CRP and ESR values were lower than those in the wild-type group. These data showed differences in the clinical presentation in both groups and that the vaccine may play a role in reducing the virulence and infectivity of the Delta variant.
Vaccines could reduce the severity of disease and accelerate virus clearance for Delta-variant-infected patients [10]. Our data confirm that the vaccine also has a significant protective effect on the Delta variant – the incidence of severe classification was significantly lower in the Delta variant group, and the risk of progression to severe classification in the unvaccinated patients was 10–15 times that of the vaccinated patients. A previous study also confirmed that the vaccine was significantly effective in preventing severe infection and death [11]. With the increased vaccine use and virus mutation, the therapeutic strategies for SARS-CoV-2 infection have shown differences in clinical practice. In the early stage, we empirically used antiviral drugs, antibiotics, and immune enhancers in the wild-type group; supportive care and symptomatic treatment were the main treatment procedures for patients infected with the Delta variant, and specific immune globulins may be used for patients with severe classification. Recent studies have revealed that SARS-CoV-2 infection may cause disorders of the coagulation mechanism and thrombosis and may be associated with a poor prognosis [12, 13]. Therefore, anticoagulant therapy is a primary therapy strategy for patients, including those with the Delta variant. In this study, the virus clearance time of patients in the Delta-variant group was significantly faster than that in the wild-type group under the synergistic effect of vaccine and drug treatment, leading to shorter hospital stays and potentially lower medical costs in patients with the Delta variant.
In conclusion, compared with patients with wild-type SARS-CoV-2, our study confirmed that patients with the Delta variant have high transmissibility and a shorter incubation period, and vaccine injection can reduce the incidence of severe classification and promote viral clearance.