Introduction
Congenital heart defects (CHD) are the most frequent of the major congenital anomalies, representing a major global health problem and are responsible for one of the highest mortalities in newborns after birth, especially if delivered preterm [1–4]. Indeed 28% of all major congenital defects consist of heart defects [2]. Reported total CHD birth prevalence increased substantially over time, from 0.6 per 1000 live births in 1930 to 1934 to 9.1 per 1000 live births after 1995. Over the last 15 years, stabilization occurred, corresponding to 1.35 million newborns with CHD every year [1]. The estimate of 8 per 1000 live births is generally accepted as the best approximation [4]. As the management of neonates with CHD is constantly improving [5–8], there is still little effort put into the optimization of planning the subsequent pregnancies. The lack of development of strategies regarding the timing of the next conception to minimize the risk of recurrence of CHD is concerning.
The best inter-pregnancy interval (IPI) for the next successful pregnancy has been thoroughly studied in the past years. Six background papers and one supplementary paper were considered by World Health Organization experts in preparing the recommendations for birth spacing to improve maternal and neonatal outcome [9–13]. They recommended in uncomplicated pregnancies after a live birth, an interval before attempting the next pregnancy of at least 24 months in order to reduce the risk of adverse maternal, perinatal and infant outcomes [14]. However, there are no data focusing on heart defects and counselling of parents in regard to what might be optimal for the next pregnancy.
The aim of the study was to determine whether IPI, maternal age, or number of pregnancies have any influence on the recurrence of congenital heart disease in the subsequent pregnancies.
Material and methods
The study was designed following the STROBE guideline statement [15] for case-control studies. Retrospective analysis of the documentation, consisting of medical histories and ultrasonographic examinations of fetuses from the reference centre of prenatal cardiology was conducted. Data included the cases that were diagnosed from years 2001 to 2017.
Data related to IPI, maternal age, number of past pregnancies, medical history, number of pregnancy losses, and type of CHD were collected. Patients who had a past history of pregnancy with congenital heart disease and who had a subsequent pregnancy with or without congenital heart disease were eligible for the study. Exclusion criteria were pregnancies conceived by in-vitro fertilization, multiple pregnancies, pregnancies with non-cardiac congenital defects and unknown or incomplete follow-up of patients and pregnancies. All eligible cases were analysed retrospectively and divided into two groups: group I – no congenital heart defect in subsequent pregnancy, group II – recurrence of congenital heart disease in following pregnancy. Smoking habits as well as socio-demographic characteristics were not evaluated in this study.
Ethical approval
All procedures followed were in accordance with ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all patients for being included in the study. All patient information were anonymised.
Ethical approval for the research was obtained from the Bioethical Committee of the Medical University of Lodz, reference number: RNN/326/18/KE.
Statistical analysis
The IPIs (birth-to-pregnancy intervals) were calculated according to the standardised method for IPI calculation [14], as the time between the resolution of primary pregnancy of a fetus with CHD and the estimated start of the subsequent pregnancy (birth date minus gestational age). Gestational age was based on the best clinical estimate using ultrasound examination and/or last menstruation period. Inter-pregnancy intervals were rounded to full months and categorised as: 1–6, 7–12, 13–24, 25–36 and > 36 months.
Other variables considered were age of the mother, number of past pregnancies, number of past pregnancy losses, past and present condition of the pregnancy.
Normality for continuous variables was assessed by the Shapiro-Wilk test. Study groups were compared using Student’s t-test or the Mann-Whitney U-test according to data distribution. The χ2, Fisher’s exact test (for expected cell-size < 5) and McNemar test (for repeated measures) were used for dichotomous variables. Multivariable Cox proportional hazard regression was used to estimate adjusted hazard ratios (AHR) and their 95% confidence interval (CI), accounting for maternal age and parity. Missing data were excluded from the analysis. Statistical analysis was performed using Microsoft Excel and Statistica 13.1 software. Two-tailed p values less than 0.05 were accepted to be statistically significant.
Results
We selected 144 cases of women with registered pregnancy with a complete medical history and echocardiographic examination, following previous pregnancies with fetuses having CHD. After dividing the women into two groups, there were 117 women without subsequent CHD (group I) and 27 women with subsequent CHD (group II).
Percentage distribution of CHD in both groups is presented in Table I. The CHD in approximately 25% of group I was hypoplastic left heart syndrome (HLHS) and in approximately 25% of group II it was ventricular septal defect (VSD). The observed differences were consistent with random variation (χ2 test p > 0.4300). Intra- and intergroup similarity between CHDs were assessed: 12 out of 27 cases were concordant CHD and 11 out of 27 cases CHD were similar. There was no agreement between the past and present CHD in the 4 cases. Concordance of defects was assessed according to the anatomical similarity. There was no statistical evidence for concordance between the past and the subsequent CHD phenotype (p > 0.4000).
Table I
Diagnoses of congenital heart defects and numbers of fetuses with CHD present
[i] HLHS – hypoplastic left heart syndrome, VSD – ventricular septal defect, d-TGA – transposition of the great arteries, TOF – tetralogy of Fallot, CoA – coarctation of the aorta, ASD primum – atrial septal defect type primum, AS – aortic stenosis, HRHS – hypoplastic right heart syndrome, IAA – interrupted aortic arch, MA – mitral atresia, PA – pulmonary atresia, PS – pulmonary stenosis, SV – single ventricle, TAPVC – total anomalous pulmonary venous connection. Complex defects were such as more elements of congenital heart defects were present in one fetus for instance – d-TGA plus pulmonary stenosis or tetralogy of Fallot with right aortic arch or Ebstein syndrome with pulmonary atresia.
Maternal age was compared between groups I and II and statistical significance of difference was not found (p > 0.1640). The studied group consisted of younger women (< 35 years), 88.03% and 74.07% accordingly.
Group II presented a significantly higher number of previous pregnancies (4 and more) than group I (≥ 4: I to II: 4.3% to 23.2%). Accordingly, group I had a significantly smaller number of pregnancies (2: I to II: 70.1% to 51.8%). The difference in number of pregnancies was statistically significant (Table II, p < 0.0010), but it may be a confounder for short IPIs.
Table II
Number of past pregnancies comparison between studied groups (Pearson’s χ2 test p < 0.0010)
| Numbers of past pregnancies | Studied groups | |||
|---|---|---|---|---|
| I (CHD…NHA) | II (CHD…CHD) | |||
| N | % | N | % | |
| 2 | 82 | 70.09 | 9 | 33.33 |
| 3 | 30 | 25.64 | 8 | 29.63 |
| 4 and more | 5 | 4.27 | 10 | 37.04 |
| Total | 117 | 100.00 | 27 | 100.00 |
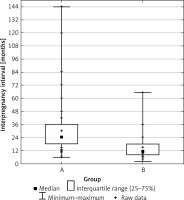
The IPIs for the groups are described in the Table III. Median inter-pregnancy intervals for absence of subsequent CHD following CHD in group I was 2 years. In group II, presence of subsequent CHD was observed in only 3 cases for the interval of > 2 years (I to II: 43.59% to 11.11%).
Table III
Inter-pregnancy interval comparison between studied groups. Median and interquartile ranges were calculated. The difference was found to be statistically significant (Student’s t-test, p < 0.0040). The continuous variable was grouped into 3 categories (1–6, 7–12, > 12 months)
The IPI for subsequent CHD was 11.11% (1–6 months) and 59.26% (7–12 months), whereas in group I the number of cases was 0.85% (1–6 months) and 12.82% (7–12 months). The difference between presence and absence of CHD of fetus and IPI was statistically significant (Student’s t-test p < 0.0040).
To assess whether the difference in IPI between groups I and II could have been a result of increased parity (> 3) in group II, the cases of pluriparity were excluded. The IPI for subsequent CHD between new groups was still significantly shorter with a median of 11 months compared to the median of 24 months for the group of healthy fetuses in subsequent pregnancy (p < 0.0001).
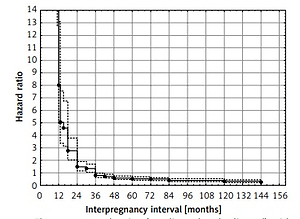
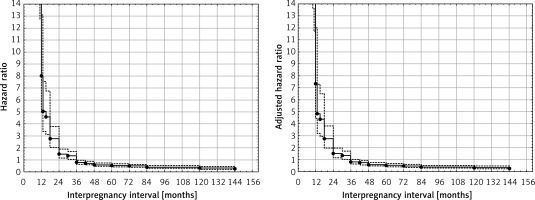
Calculated adjusted hazard ratios (AHR) for IPIs accounting for maternal age and parity are presented in Table IV. Assessment of maternal age and parity did not materially change the observed results for IPI (Figures 1 and 2).
Table IV
Results of univariable and multivariable (maternal age and parity adjusted) Cox proportional hazard regression. Inter-pregnancy intervals were divided into groups (< 12, 13–24, 25–36 and > 36 months)
Discussion
Mothers with advanced age, cold or fever, gestational diabetes, family history of CHD, presence of major extra-cardiac anomalies, maternal smoking during pregnancy, and maternal medication exposure are well-known factors connected with risk for CHD [16–18].
In our research we focused on the influence of IPI on the recurrence of CHD in subsequent pregnancies. Current literature does not provide a similar analysis and thus comparison with other studies is not possible. The IPI associated with the absence of CHD following previous pregnancies with CHD (group I) was longer than that for those with subsequent pregnancies resulting in fetuses having CHD (group II). The median interval in group I was 24 months compared to the median of 11 months in group II. The result is statistically significant (p < 0.004). The majority of women in group II (59.26%) got pregnant at 7 to 12 months after delivery of newborns with CHD.
The causes of the association of a short IPI with the recurrence of a CHD are unclear. Todoroff and Shaw suggested for neural tube defects that the increased risk associated with a short IPI might be related to inadequate replenishment of nutrients essential for normal fetal development, as a result of close successive pregnancies [19]. Others suggested explanations including the inflammatory process, due to the gap between the pregnancies reaching 24 months. Unfortunately our experimental design does not allow us to speculate on the etiologically of CHD recurrence. In the analysis we did not recognise recurrence of a single kind of CHD phenotype, which indicates that other factors rather than IPIs, such as genetics, may have a greater influence on the outcome of specific CHD. The strength of our study is in its unique population of pregnant women, showing recurrence of CHDs in following pregnancies coming from a single referral centre. A limitation was the lack of control with other potential clinical covariates that may be associated with CHD such as history of cold or fever during pregnancy, gestational diabetes mellitus, family history of congenital heart disease, maternal smoking during pregnancy and before pregnancy, and maternal medication exposure.
In a similarly designed study which analysed IPI and the risk of gastroschisis it was found that short IPI was associated with an increased risk for gastroschisis, particularly among women whose preceding pregnancy resulted in a miscarriage or termination and among those who resided in northern study areas with winter/fall conception [17]. Todoroff and Shaw found an increased neural tube defect (NTD) risk associated with an inter-pregnancy interval of < 6 months among mothers whose prior pregnancy had resulted in a live birth [18].
To our knowledge, there is no previous study focusing on the presence of congenital heart disease following a previous pregnancy in which fetuses had CHD. However, the general topic of the association of IPI with the results of subsequent pregnancies has been studied in the past years. Six background papers and one supplementary paper were considered by the World Health Organization experts in preparing the recommendations for birth spacing to improve maternal and neonatal outcomes [8, 9, 11, 12, 20, 21]. Together, the set of papers provided an extensive collection of information on the relationship between birth-spacing intervals and maternal, infant and child health outcomes. To summarize, birth-to-pregnancy intervals of 6 months or shorter are associated with elevated risk of maternal mortality. Birth-to-pregnancy intervals of around 18 months or shorter are associated with elevated risk of infant, neonatal and perinatal mortality, low birth weight, small size for gestational age, and pre-term delivery. The recommendation for the minimum interval between a live birth and attempting another pregnancy should be 24 months and after a miscarriage or induced abortion, the recommended minimum interval to the next pregnancy is at least 6 months in order to reduce risks of adverse maternal and perinatal outcomes [14].
Shachar et al. suggested lowering the current minimal IPI recommendation to only 18 months (vs. 24 months according to the latest World Health Organization recommendations), with even shorter recommended minimal IPI for women of advanced age and those who conceive after a spontaneous or induced abortion [22].
Currently, there is no consensus concerning IPI after miscarriages, with the difference varying from < 3 months to > 6 months. The researchers agreed that a safe IPI for the next pregnancy after delivery is about 18 to 24 months. Conversely, short IPI after pregnancy termination was associated with decreased odds for preterm birth, which is consistent with some recent studies [13, 23], but not all [8].
The IPI for lower risk of recurrence of CHD obtained in our analysis is longer than 24 months. It is the same interval as suggested in 2015 by the World Health Organisation; however, their report focused on the risk of prematurity, fetal death, low birth weight and small size for gestational age [14].
We speculate that IPI after a miscarriage or induced abortion in pregnancies with CHD should be the same as after live birth or 24 months. It is also worth noting that in the studied CHD we did not include any persistent ductus arteriosus and atrial septal defect, as these anomalies are not diagnosed in fetuses.
Our results expand the current data on birth spacing, thus being of great importance in clinical practice for obstetricians and gynaecologists as well as mothers who have experienced pregnancy complications, such as CHD in fetus. This problem concerns 1.35 million women every year [1], with almost 40 thousand CHD recurrences. According to the available data on birth spacing and prevalence of recurrent CHDs, IPIs of 1–24 months result in more than 95% of recurrences of CHD annually. The introduction of a longer inter-pregnancy interval (> 24 months) and providing clinicians with information on how to approach birth spacing after previous CHD of fetus, could reduce the risk of recurrent CHDs by 68.9% (an assumption based on a 71.1% rate of WHO birth-spacing recommendations fulfilment and calculated limitations of > 24 months cut-point for the studied group) [19, 24]. An increased risk for recurrence of CHD in a subsequent pregnancy is therefore not only a medical, but also a social and family problem. Our research suggests an optimal IPI for planning the next pregnancy after the CHD in previous pregnancy.
Limitations of the study include the retrospective study design and relatively small group of subsequent pregnancies with CHD after previous pregnancies with CHD. However, we were still able to demonstrate significant relationships between the groups. Nevertheless, to our knowledge this is the first investigation of IPI on recurrence risk of CHD. The potential aetiologies that may underlie this observation could not be specifically addressed due to the lack of pertinent information. Possibilities include dietary replenishment and folate repletion that could be relevant factors in planning another pregnancy after pregnancy with CHD [25, 26].
Further prospective studies with a larger number of patients and some socio-demographic characteristics controlled for dietary and folate intake are required in this regard.
In conclusion, the optimal inter-pregnancy interval to reduce the risk of recurrence of congenital heart defects is 24 months. Shorter intervals are associated with a higher risk of recurrence of congenital heart defects in the next pregnancy and are independent of the age of the woman. Parity was proven to be an important confounder of the study. Multivariable analysis including parity and maternal age did not affect the confidence intervals of hazard ratios for inter-pregnancy intervals in the study.