Introduction
Vitamin D is a well-known fat-soluble vitamin that regulates calcium homeostasis and bone metabolism, as well as a steroid prohormone with multiple pleiotropic effects on various target tissues. Vitamin D is involved in the control of cell growth, proliferation, and apoptosis and has immunomodulatory and anticoagulant effects [1–4].
Vitamin D deficiency is a common health problem affecting all age groups worldwide [1, 5]. Circulating 25-hydroxyvitamin D [25(OH)D] is the accepted marker for assessing vitamin D status [1, 3–5]. According to the Endocrine Society Clinical Practice Guidelines, 25(OH)D levels less than 20 ng/ml reflect a deficient state [1, 5]. The prevalence of hypovitaminosis D in the general population has been reported to be over 50% in large observational studies [6, 7]. Moreover, a close relationship between vitamin D deficiency and various systemic disorders associated with significant morbidity and mortality has been demonstrated in several studies [1–4, 8].
Hypovitaminosis D has also been hypothesized to be a possible risk factor for morbidity and mortality in critical illness admission [2, 4]. Studies worldwide in acutely ill adult and pediatric patients have revealed a high prevalence of hypovitaminosis D associated with poor outcomes [2, 9–12]. A prevalence of hypovitaminosis D ranging from 30% to 70% has been reported in pediatric intensive care unit (PICU) patients, and hypovitaminosis D at PICU admission has been associated with increased illness severity and mortality, and longer hospital stays [2, 10, 12–14]. Furthermore, there have been prospective studies in acute illness assessing the longitudinal trend in vitamin D levels. In this regard, significant decreases in vitamin D levels have been reported in the follow-up of both patients admitted to the intensive care unit and patients who underwent elective or semi-elective operations [15–18].
The aim of this study is to compare the vitamin D status of critically ill children with that of healthy children, monitor the vitamin D status during the stay in the PICU, and examine whether hypovitaminosis D affects patient outcomes.
Material and methods
All patients admitted to the PICU at our institution between January 2014 and October 2018 were included in the study group of this case-control study. Although the age range determined for this unit is between 1 month and 18 years, patients under 1 year of age were not included in the study due to routine use of vitamin D supplements. In addition to the patients taking vitamin D supplements within the 3 months before admission, those with chronic hepatic, renal, or metabolic diseases, endocrinopathies, and gastrointestinal malabsorption syndromes were excluded from the study. Age- and sex-matched control patients were randomly recruited from healthy children referred to the outpatient clinic for routine blood tests. All parents of each patient were informed about the details of the study and gave their written consent according to the International Ethical Guidelines and Declaration of Helsinki for the publication of clinical findings and laboratory reports. Institutional ethical and scientific committee approval for the research was obtained (approval no: 2013/24746).
Baseline data including demographic variables such as age, gender, weight, past medical history, and admission diagnosis were noted. Standard deviation scores (SDS) for weight-for-age were calculated using the Statistical Analysis Software program for the Centers for Disease and Prevention Growth Charts (2000, Centers for Disease Control and Prevention, Atlanta, GA). Two scores for illness severity were used for routine assessment following the PICU admission: the pediatric logistic organ dysfunction (PELOD) and the pediatric risk of mortality (PRISM) III scores [19, 20]. The PELOD score evaluates neurological, cardiovascular, renal, respiratory, hematological, and hepatic dysfunctions. In calculating the score, each organ dysfunction receives points, with a total score ranging from 0 to 71. The PRISM III score is based on assessing the systolic blood pressure, temperature, mental state, heart rate, blood gases, renal and hematological functions, and blood glucose. In calculating, each parameter receives points with a total score ranging from 0 to 74. In addition to serum calcium, phosphate, albumin, and CRP levels, biochemical markers used in the severity of illness scores were recorded. Clinical outcome measures including respiratory and inotropic support, sepsis, duration of PICU stay, and mortality were observed.
Patients’ serum samples from freshly drawn blood were analyzed by colorimetry for Ca, P, and albumin levels, and by immunoturbidimetry for CRP levels using an autoanalyzer (Cobas 8000, Roche Diagnostics, Basel, Switzerland). To evaluate the prospective vitamin D status of the study population, blood samples were collected on days 1, 5, and 10 in the PICU. Serum samples were separated by centrifugation and stored at –80°C before the analysis. 25(OH)D levels were measured on the LIAISON autoanalyzer (DiaSorin Inc., Stillwater, MN, USA) using the DiaSorin LIAISON 25-OH Vitamin D TOTAL assay, a chemiluminescence immunoassay (CLIA) that detects 25(OH)D2, 25(OH)D3, and other hydroxylated vitamin D metabolites in serum or plasma. Measurements were carried out and calibrated according to the manufacturer’s instructions. The range of the assay was between 4.0 and 150 ng/ml. The intra- and interassay coefficients of variation of serum samples were < 8%. 25(OH)D levels of the healthy controls were analyzed from fresh serum samples by the same autoanalyzer all year round. Serum 25(OH)D levels of 20–30 ng/ml and < 20 ng/ml were categorized as vitamin D insufficiency and deficiency, respectively [1, 5]. The effect of seasonal factors on vitamin D levels was ignored.
Statistical analysis
Before starting the study, power calculations were performed using data from several case-control studies that examined the prevalence of vitamin D deficiency in various populations [21, 22]. The power analysis determined that 26 subjects per group were needed to achieve an α of 0.05, with an effect size of 0.56, and a power of 0.80. Categorical data were expressed as percentages. Continuous data were expressed as the mean ± SD or median and interquartile range, as appropriate. The patient group was divided into three subgroups according to their initial serum vitamin D levels. We used the χ2 or Fisher’s exact test to compare categorical variables between the groups. To compare quantitative continuous variables, the independent Student’s t-test was used if the data were normally distributed, and the Mann-Whitney U or Kruskal-Wallis test was used if the variables demonstrated a non-normal distribution. The normality of the distribution for each numerical variable was preliminarily assessed by the Kolmogorov-Smirnov test. Serial measurements of vitamin D levels within a group were compared with the Greenhouse-Geisser test. Statistical significance was considered if the p-value was smaller than 0.05. Statistical analysis was performed with the IBM SPSS statistical software (SPSS v.21.0 for Mac OS X).
Results
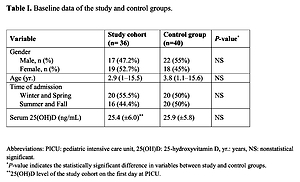
Table I shows the baseline characteristics of the study and control groups. Thirty-six PICU patients and 40 healthy children were enrolled. The median age was 2.9 (1–15.5) years in the study cohort and 3.8 (1.1–15.6) years in the control group. Seventeen patients in the study group (47.2%) and 22 individuals in the control group (55%) were male. There was no significant difference between the two groups in terms of age, sex distribution or admission season (p = 0.52, 0.49, and 0.62, respectively). The mean initial 25(OH)D level of the study group was 25.4 ±6.0 ng/ml, and it showed no significant difference (p = 0.71) from the mean 25(OH)D level of the control group (25.9 ±5.8 ng/ml). The prevalence of vitamin D insufficiency and deficiency in the study group was found to be 55.5% (20/36) and 16.6% (6/36), respectively. Ten patients (27.7%) were found to have adequate serum 25(OH)D levels.
Table I
Baseline data of the study and control groups
| Variable | Study cohort (n = 36) | Control group (n = 40) | P-value* |
|---|---|---|---|
| Gender, n (%): | |||
| Male | 17 (47.2%) | 22 (55%) | NS |
| Female | 19 (52.7%) | 18 (45%) | |
| Age [years] | 2.9 (1–15.5) | 3.8 (1.1–15.6) | NS |
| Time of admission: | |||
| Winter and spring | 20 (55.5%) | 20 (50%) | NS |
| Summer and fall | 16 (44.4%) | 20 (50%) | |
| Serum 25(OH)D [ng/ml] | 25.4 ±6.0** | 25.9 ±5.8 | NS |
Table II shows the clinical characteristics and outcome measures. The median SDS of weight for age was –0.7 (range: –5.2–3.7). Leading causes of PICU admission were infections (19/36, 52.7%) and neurological monitoring (7/36, 19.4%). Respiratory support was applied to 23 (63.8%) patients. Seventeen (47.2%) patients received inotropic/vasopressor support. Sepsis/septic shock was encountered in 4 (11.1%) patients.
Table II
Clinical characteristics and their statistical comparisons based on vitamin D status of the study cohort at the time of PICU admission
| Variable | Total study cohort (n = 36) | Vitamin D-sufficient (≥ 30 ng/ml) (n = 10) | Vitamin D-insufficient (20–30 ng/ml) (n = 20) | Vitamin D-deficient (≤ 20 ng/ml) (n = 6) | P-value* |
|---|---|---|---|---|---|
| Serum 25(OH)D [ng/ml] | 25.4 ±6.0 | 31.4 ±1.2 | 25.6 ±2.9 | 15.0 ±4.6 | |
| Gender, n (%): Male | 17 (47.2) | 5 (50) | 9 (45) | 3 (50) | NS |
| Age [years] | 2.9 (1–15.5) | 6 (1–15.5) | 1.6 (1–14.5) | 4.1 (1.1–11.5) | NS |
| Time of admission, n (%): | NS | ||||
| Winter and spring | 20 (55.5) | 4 (40) | 13 (65) | 3 (50) | |
| Summer and fall | 16 (44.4) | 6 (60) | 7 (35) | 3 (50) | |
| Admission category, n (%): | |||||
| Infection | 19 (52.7) | 3 (30) | 10 (50) | 6 (100) | 0.02 |
| Neurologic monitoring | 7 (19.4) | 2 (20) | 5 (25) | 0 (0) | NS |
| Postoperative | 5 (13.8) | 3 (30) | 2 (10) | 0 (0) | NS |
| Respiratory failure | 2 (5.5) | 1 (10) | 1 (5) | 0 (0) | NS |
| Other | 3 (8.3) | 1 (10) | 2 (10) | 0 (0) | NS |
| Weight (SDS) | –0.7 (–5.2–3.7) | –0.5 (–3.6–3.6) | –0.9 (–5.2–3.7) | –2.1 (–3.7–1.6) | NS |
| PELOD score | 10 (0–41) | 2 (0–21) | 10 (0–31) | 16 (0–41) | NS |
| PRISM III score | 10 (0–25) | 6 (0–20) | 10.5 (0–25) | 8.5 (4–24) | NS |
| Serum calcium (N: 8.4–10.8 mg/dl) | 9 (6–10.8) | 9.6 (7.1–10.8) | 8.9 (6–10.6) | 8.5 (7.3–9.6) | NS |
| Serum phosphate (N: 2.7–5.5 mg/dl) | 4 (1.7–9.2) | 4.2 (3.6–9.2) | 3.8 (1.7–5.8) | 3.7 (2.8–5.4) | NS |
| Serum albumin (N: 3.2–5.4 g/dl) | 3.7 (1.4–5.1) | 4.2 (2.7–4.7) | 3.6 (2.2–5.1) | 2.8 (1.4–5.1) | 0.05 |
| CRP Elevated (≥ 0.5 mg/dl), n (%) | 25 (69.4) | 5 (50) | 15 (75) | 5 (83.3) | NS |
| Respiratory support, n (%) | 23 (63.8) | 6 (60) | 12 (60) | 5 (83.3) | NS |
| Inotropic/vasopressor support, n (%) | 17 (47.2) | 4 (40) | 9 (45) | 4 (66.6) | NS |
| Sepsis, n (%) | 4 (11.1) | 0 (0) | 2 (10) | 2 (33.3) | NS |
| PICU stay [days] | 17.5 (5–161) | 14 (9.3–66) | 14.5 (5–68) | 30 (10–161) | NS |
| Hospital mortality, n (%) | 17 (47.2) | 4 (40) | 10 (50) | 3 (50) | NS |
Statistical comparisons regarding clinical characteristics between vitamin D subgroups according to initial vitamin D levels are also shown in Table II. Patients were divided into sufficient (n = 10), insufficient (n = 20), and deficient (n = 6) subgroups. No significant difference was found between vitamin D subgroups in the baseline characteristics including demographic variables, admission season, two scores for illness severity (PELOD and PRISM III), and biochemical markers including serum calcium, phosphate, and CRP levels. The median level of serum albumin, which was 3.7 g/dl (range: 1.4–5.1) in the total cohort, was significantly lower (p = 0.05) in patients with vitamin D deficiency (2.8 g/dl, range: 1.4–5.1), compared to the vitamin D sufficient subgroup (4.2 g/dl, range: 2.7–4.7). Regarding the total cohort, there was no difference (p = 0.31) in vitamin D levels between patients hospitalized for infectious diseases (25(OH)D = 24.5 ±7.0 ng/ml) and patients with other admission diagnoses (25(OH)D = 26.5 ±4.7 ng/ml). The subgroup with vitamin D deficiency consisted of patients hospitalized for infectious diseases, whereas this admission category was observed in 50% and 30% of patients with vitamin D insufficiency (p = 0.02), and sufficiency (p = 0.01), respectively. Although higher respiratory support and vasoactive drug requirement, higher prevalence of sepsis, and longer PICU stay were observed in the subgroup with vitamin D deficiency, no statistically significant difference was present for these variables compared to sufficient and deficient subgroups. A total of 17 (47.2%) patients died in the PICU. Although their mean initial serum 25(OH) D level was lower than that in patients who survived, the difference was not statistically significant (24.1 ng/ml vs. 26.6 ng/ml, p = 0.22). The mortality rate was also not different between vitamin D subgroups.
Serial measurements of serum 25(OH)D levels were not possible in 3 patients in the study group, as 2 patients died and 1 patient was discharged before the 5th PICU day. Serial 25(OH)D levels in 33 patients in the cohort on days 1, 5, and 10 are shown in Table III. 25(OH)D levels were 24.5 ±5.7 ng/ml and 23.6 ±5.8 ng/ml on days 5 and 10, respectively. The decrease in 25(OH)D levels was found to be statistically significant (p < 0.001). The prevalence of vitamin D insufficiency, which was 55.5% (20/36) on the 1st PICU day, was found to be 63.6% (21/33) on the 10th day. Correspondingly, the prevalence of vitamin D deficiency increased from 16.6% (6/36) on the 1st day to 27.2% (9/33) on the 10th day.
Table III
Serial serum 25(OH)D (ng/ml) levels of the cohort at admission, on 1st, 5th, and 10th days in PICU
| PICU days | N | Serum 25(OH)D [ng/ml] | P-value* |
|---|---|---|---|
| 1st day | 33 | 25.7 ±5.9 | < 0.001 |
| 5th day | 33 | 24.5 ±5.7 | |
| 10th day | 33 | 23.6 ±5.8 |
Discussion
In the current study, the prevalence of hypovitaminosis D was found to be high in the patient cohort at admission, and a decrease was observed in the serial vitamin D measurements of the patients. Despite the initial high prevalence of hypovitaminosis D in the patient group, there was no difference between the mean vitamin D levels of the patient and control groups, unlike some case-control studies [21, 22]. No association was found between initial vitamin D levels and sepsis, scores for illness severity, hospital stay, or mortality in the study group.
A meta-analysis of 17 eligible studies on the prevalence of hypovitaminosis D in pediatric critical illness and its relationship to clinical outcomes revealed that 54% of critically ill children had deficient 25(OH)D levels (< 20 ng/ml) at PICU admission [2]. The total prevalence of hypovitaminosis D at PICU admission in our study was similar, and the mean 25(OH)D level in our cohort was 25.4 ±6.0 ng/ml, indicating vitamin D insufficiency. Serum albumin levels were significantly lower in our patients with vitamin D deficiency. The current knowledge on vitamin D transport in blood is that about 88% of serum 25(OH)D is bound to vitamin D binding protein (VDBP), 10% is bound to albumin and less than 1% is in the free state [3, 23]. Therefore, serum albumin levels can affect serum 25(OH)D levels, as shown in our study. Jhang et al. [13] compared the patient groups with adequate and inadequate vitamin D levels and observed marked differences in age and weight at PICU admission. In their study, hypovitaminosis D was further associated with low serum albumin levels and pediatric organ dysfunction scores. Demographic variables and severity of illness scores were not different between vitamin D subgroups in our study, which may be due to the small size of our cohort.
Vitamin D acts in the regulation of innate immune cells and defense against infectious agents through the activation of toll-like receptors in the host cell and the production of cathelicidin, an antimicrobial peptide [1, 3, 4]. Meta-analyses evaluating vitamin D levels in critically ill adults and children have suggested an association between vitamin D deficiency and a potential increased risk of developing severe infections including coronavirus disease 2019 [9–11]. Children with vitamin D deficiency were found to have a higher risk of sepsis than children without vitamin D deficiency in a recent meta-analysis involving 2382 children [10]. In the study of Madden et al. [14], although 25(OH)D levels were not lower in PICU patients hospitalized for infectious diseases compared to other patients, patients presenting with severe septic shock had markedly lower levels. In our study, although the subgroup with vitamin D deficiency consisted of patients hospitalized for infectious diseases, the mean 25(OH)D level was not different between the patients hospitalized for infectious diseases and the patients with other admission diagnoses. We did not find any association between vitamin D levels and blood culture positivity.
The meta-analysis by McNally et al. [2] revealed that vitamin D deficiency was associated with greater illness severity, multiple organ dysfunction, and mortality in the PICU. In the current study, although higher respiratory support and vasoactive drug requirement, higher prevalence of sepsis, and longer PICU stay were observed in patients with baseline deficient 25(OH)D levels, no significant difference was found for these variables compared to the sufficient and deficient subgroups. The mortality rate was not different between vitamin D subgroups. The difference in vitamin D levels between surviving and non-surviving patients in the study group was not statistically significant.
The alterations in vitamin D levels in acute illness have been examined in some studies evaluating levels during a condition associated with inflammation or pre- and postoperative serum 25(OH)D levels [15–17, 24, 25]. In the current study, a gradual decrease in 25(OH)D levels of the study group, which was statistically significant compared to the admission levels, was detected on the 5th and 10th days in the PICU. Acute decreases in serum 25(OH)D levels have been reported in children after cardiac surgery, and lower vitamin D levels have been associated with worse clinical outcomes [15, 16]. In the study of Higgins et al. [18], a significant decrease in 25(OH)D levels was present over the first 3–10 days in the ICU. They stated that the acute decrease in 25(OH)D might be related to the possible change in VDBP. Blomberg Jensen et al. [25] reported that postoperative levels of serum VDBP and total 25(OH)D were unaltered, whereas Reid et al. [17] observed a significant decrease in serum 25(OH)D, VDBP, albumin concentrations, and the molar ratio of 25(OH)D to VDBP in adult patients with an inflammatory response 1 day after knee surgery. Although the postoperative decrease in total 25(OH)D levels can be explained by the loss of VDBP and albumin as part of the acute phase response in the study of Reid et al. [17], after 3 months 25(OH)D levels were still significantly lower than preoperative levels, despite improved VDBP and albumin values. Similarly, Madden et al. [24] observed lower serum VDBP and total 25(OH)D levels in critically ill children compared to healthy children and reported that low VDBP levels increased the bioavailability of 25(OH)D. Therefore, it has been hypothesized that serum 25(OH)D levels alone might not be a reliable indicator of vitamin D status in acute illness, as they do not directly indicate the bioavailability of 25(OH)D [23, 24]. Further studies are needed to clarify optimal serum 25(OH)D cutoff levels associated with optimal vitamin D functionality.
The main limitations of this study were the sample size and patients’ heterogeneity in terms of disease type and severity. The small sample size in this single-center study limited the capacity to analyze specific subgroups of patients; therefore our results may not be generalizable. Although the CLIA method used in our study has been accepted as a reliable immunoassay method in the measurement of total serum 25(OH)D, the fact that the reliability of immunoassay methods is lower at very high and very low 25(OH)D values is a further limitation [26]. The 25(OH)D cutoff values we used were determined for the general population and may not be applicable to critically ill patients. The reduction in 25(OH)D levels may be partially related to confounding causes such as dietary changes and limited sun exposure during the PICU stay. Furthermore, VDBP and parathyroid hormone levels were not measured. Further PICU studies with larger sample sizes are needed to evaluate all components of the vitamin D axis in acute illness, which may be associated with positive patient outcomes during the PICU stay.
In conclusion, due to its pleiotropic effects, vitamin D is a biomarker associated with several clinical conditions, including critical and acute illness. This study demonstrates that hypovitaminosis D at PICU admission is common and becomes more prevalent during the PICU stay. We recommend screening the longitudinal trend of serum 25(OH)D levels during the stay in the PICU. Further studies regarding bioavailable vitamin D in critically ill patients may assist in a more accurate interpretation of serum 25(OH)D levels in acute illness.