Introduction
Globally, hepatitis B virus (HBV) chronic infection of the liver is a major health issue with about 3 million cases that may progress to fulminant liver failure, cirrhosis or hepatocellular carcinoma, accounting for over 850,000 deaths, annually [1, 2]. About two-thirds of the world population lives in high-endemic regions in Asia, Africa and the Middle East, including Saudi Arabia [1]. Though there are highly protective vaccines and efficacious direct-acting nucleoside analogs, the emergence of vaccine escape and drug-resistant HBV mutants remains a clinical challenge [3, 4]. In the last couple of decades, a range of herbal formulations and bioactive phytochemicals, such as alkaloids, flavonoids, polyphenols, terpenoids, terpenes, lignans, coumarins, saponins, xanthins and anthraquinones, have been reported to have potential activity against various genetically diverse viruses, including HBV, with no issue of drug resistance [5–8]. In line with this, we have also reported various anti-HBV medicinal plant extracts and isolated compounds for their promising activity in vitro [9–15].
Ilex paraguariensis (family: Aquifoliaceae) dry leaves known as yerba mate is popularly consumed as a health-protective brewed herbal tea in Brazil, Argentina, Paraguay and Uruguay [16, 17]. Mate tea is also prepared in cold water, called tereré, which is mainly consumed in Paraguay. Mate has been reported for its antioxidant [18–20], hepatoprotective and hypocholesterolemic [17], cardiovascular protective [21], and anti-obesity [22] effects in vitro and in vivo. Further pre-clinical studies have expanded its antioxidant [23] and hypocholesterolemic [24] properties. Moreover, I. paraguariensis extract has been shown to have antifungal activity in humans [25]. Of the various bioactive compound identified in I. paraguariensis green extracts, the most abundant are caffeic acid, chlorogenic acid, dicaffeoylquinic acid, caffeine, theobromine, rutin, quercetin, and kaempferol [26]. Notably, in recent years, we have reported antiviral rutin, quercetin and kaempferol and their derivatives for their in vitro anti-HBV potential [12–15]. In this study therefore, we investigated the in vitro anti-HBV efficacy of I. paraguariensis and its bioactive phytochemicals in a HBV-reporter cell culture model, validated by high performance liquid chromatography and molecular docking.
Material and methods
Sample collection and preparation of extract and fractions
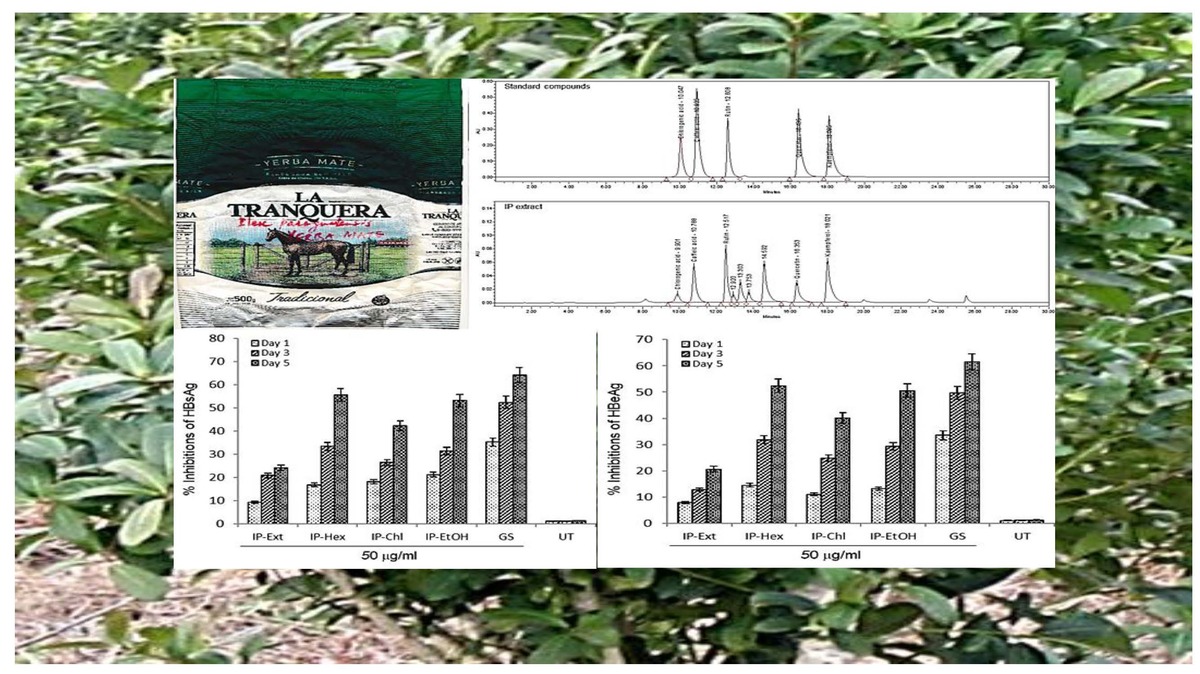
I. paraguariensis dried leaves (yerba mate) were obtained as a commercially packaged product (La Tranquera, Barcode: 17790480000371) from Argentina. All analytical grade solvents for extraction and fractionation were procured from Sigma-Aldrich (Merck KGaA, Germany). A portion (2.0 g) of powdered leaves (350.0 g) was extracted with 90% ethanol at room temperature (RT) and concentrated under reduced pressure to yield total ethanol extract (IP-Ext; 129.5 mg). The remaining portion (348.0 g) was further extracted in different solvents to furnish n-hexane (IP-Hex; 293.0 mg), chloroform (IP-Chl; 7.0 g), ethyl acetate (IP-EtAc; 10.0 mg) and ethanol (IP-EtOH; 13.5 mg) fractions by distillation under reduced pressure.
Human liver cell cultures, solvents and drugs
Human hepatoma cells HepG2 and its derivative line HepG2.2.15 (generous gift from Dr. S. Jameel, International Center for Genetic Engineering & Biotechnology, New Delhi, India) were grown in DMEM medium (Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1x antibiotic-antimycotic mix (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C with 5% CO2. For the experiments, cells (0.5 × 105 cells/100 µl/well) were grown in flat-bottom 96-well culture plates (Corning, USA) overnight. The pure natural compounds (rutin, quercetin, kaempferol, caffeic acid and chlorogenic acid) were procured from Sigma-Aldrich (Merck KGaA, Germany). Dimethyl sulfoxide (0.1% DMSO) acted as a vehicle or negative control, whereas the anti-HBV active Guiera senegalensis (GS) dichloromethane fraction [9] and the anti-HBV drug lamivudine (LAM; nucleoside analog) served as positive controls. The experiment was repeated twice for reproducibility.
Hepatocyte viability assay of I. paraguariensis preparations
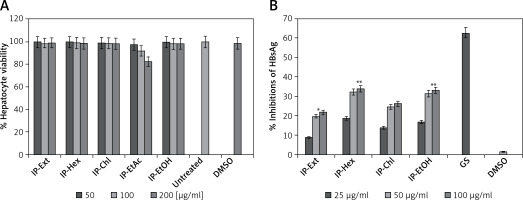
The effect of I. paraguariensis extract (IP) and fractions (IP-Hex, IP-Chl, IP-EtAc and IP-EtOH) on the viability or toxicity of HepG2 cells was assessed. The factions were prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich, Merck KGaA, Germany) and then reconstituted in DMEM to furnish four test concentrations or doses of 25, 50, 100 and 200 µg/ml. The overnight grown HepG2 cells (96-well plate) were replenished with fresh media containing different test doses of the preparations, including a negative control (0.1% DMSO) in triplicate, and incubated at 37°C for 3 days. The established assay utilizing dimethylthiazolyl diphenyltetrazolium bromide (MTT) was employed (TACS MTT Cell Proliferation Assay Kit; Tervigen, MD, USA). Briefly, after treating with MTT and incubation, the optical density (λ = 570 nm) of the samples was recorded using a microplate reader (ELx800; BioTek Instruments, Inc., VT, USA). A non-linear regression analysis was performed (Excel software 2010; Microsoft Corp., WA, USA) to determine the hepatocytes’ viability or toxicity of the preparations in relation to the negative control.
HBV envelop/surface antigen (HBsAg) inhibition assay of I. paraguariensis preparations
Dose-dependent inhibition of HBsAg expression by the selected doses of the noncytotoxic preparations (25, 50 and 100 µg/ml; in triplicate) was performed. Briefly, overnight grown HepG2.2.15 cells (96-well plate) were replenished with fresh media with treatment doses, including negative and positive controls and incubated at 37°C until day 2 (single time-point). The HBsAg production in culture supernatants was analyzed quantitatively using the diagnostic ELISA kit (cat. no. 72348; Monolisa HBsAg ULTRA, Bio-Rad Laboratories Inc., CA, USA) following the kit’s manual. The optical density (λ = 450 nm) of the samples was recorded, and analyzed (Excel) in relation to the negative control, and the activity was compared with the positive control. Further, with the determined optimally active dose, a time-course analysis of HBsAg expression was carried out. Briefly, HepG2.2.15 cells were replenished with fresh media containing 50 µg/ml (in triplicate) of each fraction, including controls, and incubated at 37°C. The culture supernatants were collected on days 1, 3 and 5, and analyzed as mentioned above.
HBV pre-core antigen (HBeAg) inhibition assay of I. paraguariensis preparations
The preparations (50 µg/ml, in triplicate) were further assessed for their time-course (days 1, 3 and day 5) inhibition of HBeAg production in the collected supernatants using ELISA kit (cat. no. KAPG4BNE3; HBeAg/Anti-HBe Elisa Kit, DIAsource ImmunoAssays; Louvain-la-Neuve, Belgium) following the kit’s manual. The optical density (λ = 450 nm) of the samples was recorded, and analyzed (Excel) as above, and the activity was compared with the positive control.
High-performance liquid chromatography (HPLC) analysis of I. paraguariensis extract
The I. paraguariensis extract was subjected to quantitative HPLC analysis for the known anti-HBV active compounds on the Alliance chromatographic system, equipped with dual wavelength absorbance detectors (Waters Instruments, Inc., MA, USA). Reverse-phase chromatography was achieved using a C18 column (4.6 × 300 mm) under gradient flow (1 ml/min) of the mobile phase (Solution A: formic acid : water; 1 : 99, v/v) and Solution B: acetonitrile : methanol; 70 : 30, v/v). The formulized gradient was 7% Solution B until 4 min and then modified to obtain 65%, 90%, 100%, and 7% Solution B at 12, 20, 23 and 27 min, respectively, as described elsewhere with minor modifications. Samples (20 µl, each) were membrane-filtered (0.45 µm filter, Millipore, USA) before injecting into the system. The identification and detection of the compounds were monitored (λ =366 nm) by comparing their retention times with those of the commercial standards.
Anti-HBV assessment of identified flavonoids and polyphenols
The HPLC identified flavonoids and polyphenols (10 µg/ml, in triplicate) were also tested for optimal suppression of HBsAg and HBeAg production in treated HepG2.2.15 cells at day 5 (single time-point) as mentioned above. LAM (2 µM) and DMSO (0.1%) served as the standard (positive) and negative control, respectively, as described previously [9, 10]. The data were analyzed (Excel) in relation to the negative control and the activity was compared with the positive control.
Molecular docking analysis
Docking of the polyphenols (caffeic acid and chlorogenic acid)with in-house modelled drug-sensitive HBV wild-type polymerase (POLwt; catalytic YMDD motif) as well as drug-resistant mutant polymerase (POLmut; catalytic ‘YIDD’ motif) proteins [10, 11] was performed using Autodock 4.2. The target proteins were prepared by removing water molecules or any unwanted bound atoms, by adding hydrogen atoms and denoting Kollman charges. The 3D structures of POL were finally energy minimized using MMFF (Merck Molecular Force Field). The 2D structures of caffeic acid (CID_689043), and chlorogenic acid (CID_1794427) were obtained from PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and prepared for docking by denoting bond orders and angles. Further, Gasteiger partial charges were defined and the energies were minimized using Universal Force Field as described elsewhere [10, 11].
The structure-based virtual docking was done inside a defined grid box by selecting the reported key amino acid residues. For both POLwt and POLmut proteins, grid boxes were adjusted to 27.1 × 26.5 × 27.9 Å, centered at 47.8 × 30.1 × 34.4 Å with 0.375 Å spacing. Lamarck Genetic Algorithm and Solis-Wets methods were employed for the global and local search, respectively, as described elsewhere [10, 11].
For each run, a total of 2.5 × 106 energy calculations was estimated, and a total of 10 docking runs was completed. Further, the population size (= 150), translational step (= 0.2), quaternions (= 5) and torsions (= 5) were adjusted. The van der Waals’ and electrostatic parameters were estimated using distance-dependent dielectric function. Finally, the docking or binding affinity (Kb) of ligands for respective target was estimated from docking or binding energy (∆G) using the formula ∆ΔG = –RT ln Kb (R = universal gas constant; 1.987 cal mol–1 K–1 and T = temperature; 298 K) as mentioned elsewhere [27].
Statistical analysis
Data analysis was performed using SPSS 17.0 (SPSS Inc., Chicago, USA). Triplicated data (mean ± S.E.M) of test samples and the positive control in relation to the negative control were analyzed by one-way ANOVA, following Dunnett’s test. P < 0.05 was considered to indicate a statistically significant difference (sample vs. positive control).
Results
Assessment of in vitro hepatotoxicity of I. paraguariensis preparations
The I. paraguariensis preparations IP-Ext, IP-Chl, IP-Hex and IP-EtOH were non-hepatotoxic up to the maximal tested dose (200 μg/ml). However, IP-EtAc had mild toxicity between 100 and 200 μg/ml doses (Figure 1 A), and therefore was excluded from antiviral analysis.
Figure 1
I. paraguariensis leaves (yerba mate). A – MTT assay showing effects of I. paraguariensis extract (IP-Ext) and its hexane (IP-Hex), chloroform (IP-Chl), ethyl acetate (IP-EtAc) and ethanol (IP-EtAc) fractions on HepG2 cell viability in relation to negative control (0.1% DMSO). Data are presented as mean ± standard error of triplicated values of each sample. **P < 0.01 and ***P < 0.001 vs. untreated control (UT). B – Anti-HBV assay showing dose-dependent inhibitions of HBV s antigen (HBsAg) by IP-Ext, IP-Hex, IP-Chl, IP-EtAc and IP-EtOH in relation to negative control (0.1% DMSO) in HepG2.2.15 cells at day 2 (single time-point). Data are presented as mean ± standard error of triplicated values of each sample. **P < 0.01 and ***p < 0.001 vs. positive control (GS)
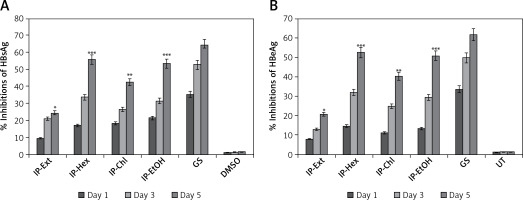
Dose- and time-dependent inhibition of HBsAg by I. paraguariensis preparations
Of the tested variable doses of I. paraguariensis non-cytotoxic preparations, the 50 μg/ml dose maximally inhibited HBsAg production, which was not significantly affected at 100 μg/ml (Figure 1 B). Therefore, 50 μg/ml was selected for further time-course inhibition analysis. Of the tested preparations, IP-Ext and IP-Chl showed mild (24.2%) and moderate (HBsAg: 42.3%) activity, respectively, whereas IP-Hex (55.6%) and IP-EtOH (53.2%) showed high activity at day 5 (Figure 2 A). Because the extended incubation of the treated cultures resulted in cell overgrowth and subsequent apoptosis (data not shown), the experiment was concluded on day 5.
Time-dependent inhibition HBV replication by I. paraguariensis preparations
HBeAg, the processed product of HBV pre-core protein co-translated with core/capsid protein by a bicistronic mRNA, is a serological gold marker of HBV DNA replication. In line with HBsAg inhibitory activity, while IP-Ext and IP-Chl had mild (20.6%) and moderate (HBsAg: 40.1%) effects, respectively, both IP-Hex (52.4%) and IP-EtOH (50.2%) had high HBeAg inhibition at day 5 (Figure 2 B). Because the extended incubation of the treated cultures resulted in cell overgrowth and subsequent apoptosis (data not shown), the study was concluded on day 5.
Figure 2
Time-dependent anti-HBV assay of I. paraguariensis extract (IP-Ext) and its hexane (IP-Hex), chloroform (IP-Chl), ethanol (IP-EtOH) fractions in HepG2.2.15 cells. A – Inhibition of HBV s antigen (HBsAg) in relation to negative/untreated control (DMSO/UT). B – Inhibition of HBV e antigen (HBeAg) in relation to negative/untreated control (DMSO/UT). Data are presented as mean ± standard error of triplicated values of each sample. **P < 0.01 and ***p < 0.001 vs. positive control (GS)
HPLC validation of anti-HBV active compounds in I. paraguariensis extract
Our quantitative HPLC analysis detected known anti-HBV active flavonoids (rutin: 18.98, quercetin: 6.52 μg/g and kaempferol: 9.10 μg/g) and polyphenols (caffeic acid: 11.43 μg/g and chlorogenic acid: 3.22 μg/g) in the extract (Figure 3; Table I).
Figure 3
HPLC chromatograms showing standard compounds (rutin, quercetin, kaempferol, caffeic acid and chlorogenic acid) and their identification in I. paraguariensis (IP extract)
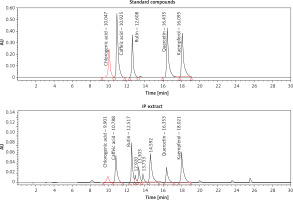
Table I
HPLC profile of anti-HBV flavonoids and polyphenols in I. paraguariensis extract
| Compounds | Structures | Retention time | Conc. ± SD* [mg/g] | |
|---|---|---|---|---|
| Flavonoids | Rutin | 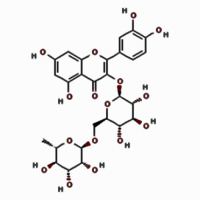 | 12.517 | 18.98 ±3.23 |
| Kaempferol | 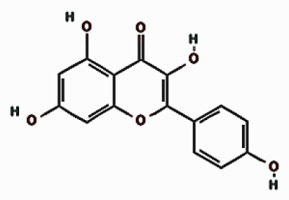 | 18.021 | 9.10 ±4.60 | |
| Quercetin | 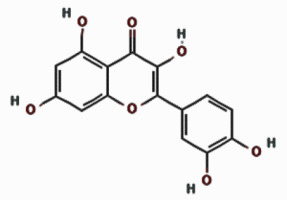 | 16.353 | 6.52 ±4.96 | |
| Polyphenols | Caffeic acid | 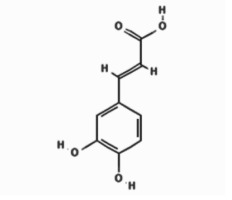 | 10.788 | 11.43 ±4.13 |
| Chlorogenic acid | 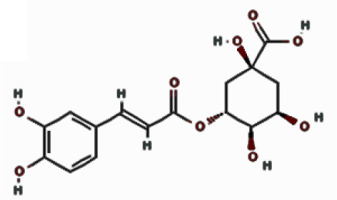 | 9.901 | 3.22 ±5.04 | |
Anti-HBV activity of identified flavonoids and polyphenols
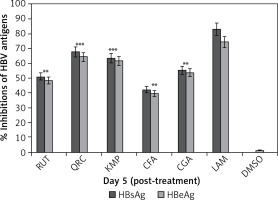
The anti-HBV activity levels of HPLC identified phytochemicals (10 μg/ml, each) on day 5 were as follows: quercetin (HBsAg: 67.8% and HBeAg: 64.4%), kaempferol (HBsAg: 63.5% and HBeAg: 61.6%), chlorogenic acid (HBsAg: 55.2% and HBeAg: 53.8%), rutin (HBsAg: 51.2% and HBeAg: 48.4%), and caffeic acid (HBsAg: 42.2% and HBeAg: 39.5%). LAM (standard) suppressed HBsAg and HBsAg by 83.2 and 74.4%, respectively (Figure 4).
Figure 4
Anti-HBV assay showing optimal inhibition of HBsAg and HBsAg production by I. paraguariensis antiviral compounds (10 µg/ml, each): rutin (RUT), quercetin (QRC), kaempferol (KMP), caffeic acid (CFA) and chlorogenic acid (CGA) in relation to negative control (DMSO) in HepG2.2.15 cells. Data are presented as the mean ± standard error of triplicated values of each sample. **P < 0.01 and ***p < 0.001 vs. positive control (LAM; 2 µM)
Interaction of polyphenols with POLwt
Because the molecular docking representations of POLwt (YMDD motif) and POLmut (YIDD motif) with lamivudine (standard ligand) have been described previously [10, 11], they were not included in the present study. Molecular docking revealed interactions of the polyphenols caffeic acid and chlorogenic acid with drug-sensitive POLwt active-site residues (Figure 5 A; Table I). Caffeic acid formed three conventional hydrogen bonds with GLY151, GLN267, and LYS239 (Figure 5 B; left). In addition, it established electrostatic interactions with LYS241 and ASP83 as well as hydrophobic interactions with LYS241 (Figure 5 B; left). Notably therein, the catalytic YMDD’ motif residue ASP206 along with other SER81, LEU82, VAL207, THR240, ASN248, PHE249, MET250, and TYR252 further stabilized the POLwt-caffeic acid complex through van der Waals’ interactions. The binding energy and binding affinity of the complex were estimated to be –6.2 kcal/mol and 3.53 × 104 M–-1, respectively (Table II).
Figure 5
Molecular docking of antiviral polyphenols with drug-sensitive HBV polymerase (POLwt) active residues. A – 3D representation caffeic acid (pink) and chlorogenic acid (blue) interactions. B – 2D representation caffeic acid (pink) and chlorogenic acid (blue) interactions
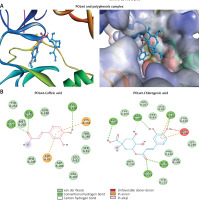
Table II
Molecular interaction of HBV polymerases (wild-type and mutant) with I. paraguariensis caffeic acid and chlorogenic acid
Chlorogenic acid established conventional hydrogen bonds with LYS241, GLY251, and GLN267 as well as the YMDD motif residue ASP206 (Figure 5 B; right). In addition, a carbon-hydrogen bond with ASP206 was also formed. Further, two electrostatic interactions with LYS241 and ASP83 along with a hydrophobic interaction with LYS241 also stabilized the POLwt-chlorogenic acid complex. Moreover, the complex was also stabilized by van der Waals’ interactions with YMDD motif residue TYR203 as well as SER81, LEU82, VAL207, LYS239, THR240, ASN248, PHE249, MET250 and TYR252. The binding energy and docking affinity of the complex were estimated to be –7.3 kcal/mol and 2.26 × 105 M–1, respectively (Table II).
Interaction of polyphenols with POlmut
Molecular docking analysis of the polyphenols suggested that both occupied the active site of the drug-resistant POLmut (Figure 6 A; Table II). Caffeic acid primarily formed conventional hydrogen bonds with GLY251, SER81, and LYS239 (Figure 6 B; left). It also had an electrostatic and a hydrophobic interaction with ASP83 and LYS241, respectively. Further, interactions of the mutant YIDD motif ASP206 residue along with LEU82, THR240, ASN248, PHE249 and MET250 through van der Waals’ stabilized the POLmut-caffeic acid complex. Notably therein, GLN262 interacted unfavorably with caffeic acid. The binding energy and binding affinity of the complex were calculated to be –5.6 kcal/mol and 1.28 × 104 M–1, respectively (Table II).
Figure 6
Molecular docking of antiviral polyphenols with drug-resistant HBV polymerase (POLmut) active residues. A – 3D representation caffeic acid (pink) and chlorogenic acid (blue) interactions. B – 2D representation caffeic acid (pink) and chlorogenic acid (blue) interactions
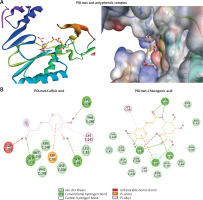
Chlorogenic acid formed conventional hydrogen bonds with POLmut residues LYS32:HZ1, LYS32, ASN36, ASN36, LYS239, LYS239 and ASP83 (Figure 6 B; right). In addition, it also had a C-H bond with SER85, an electrostatic interaction with ASP83, and a hydrophobic link with LYS241. Moreover, van der Waals’ interactions were also formed by ASN33, GLU39, ARG41, SER81, LEU82, VAL84, ALA86, ASP206, ASN236, THR240, ASN248, and PHE249 further stabilized the POLmut-chlorogenic acid complex. Notably therein, LYS239 was engaged in an unfavorable interaction. The binging energy and docking affinity of the complex were calculated to be –6.7 kcal/mol and 8.21 × 104 M–1, respectively (Table II).
Discussion
In the recent decades, numerous studies on the pharmacognosy, phytochemistry and pharmacology of natural or herbal products have driven a growing focus on antiviral drug discovery, targeting viral or host factors. Generally, consumption of such therapeutic products is believed to be more effective and safer than conventional or prescription medications. In recent times, however, acute liver failure and adverse clinical effects of some traditional Chinese medicines (TCM) and others used to treat chronic hepatitis B have been widely reported [28, 29]. Of these, herbal formulations such as Chuan Lian Zi (Meliatoosendan), Gan Cao (Glycyrrhiza uralensis, Glycyrrhiza glabra, Gan Cao Zhi, Shen Nong Ben Cao Jing, and Zhi GanCao), Ji Guo Cao (Abrus cantoniensis, Ji Gu Cao Wan), Jue Ming Zi (Cassia obtusifolia, Senna obtusifolia, Cao Jue Ming), Zexie (Alisma orientalis), and Zhen Chu Cao (Phyllanthus urinaria) have shown significant liver toxicity in some individuals [30]. In addition, some hepatitis B patients were also found to be at higher risk for developing liver toxicity or necrosis upon consuming the poly-herbal formulation Long Dan Xie Gan Tang (Akebia quinata, Alisma plantago, Angelica sylvestris, Bupleurum chinese, Gardenia jasminoides, Gentiana lutea, G. uralensis, Rehmannia glutinosa, and Scutellaria baicalensis in China [31], and Bai Fang (Angelica sinensis, Cyperus rotundus, Panax ginseng, Ligusticum wallichii, Paeonia alba, and Rehmannia glutinosa) in the United States [32].
Therefore, potential medicinal plant extracts, formulations or isolated compounds need in-depth studies for their efficacy, safety, purity, standardization and therapeutic validation. In view of this, to rule out the known organ or liver toxicity associated with some herbal products, we first tested the hepatotoxicity of I. paraguariensis preparations in cultured HepG2 cells. Although the I. paraguariensis total ethanol extract and organic fractions were nontoxic, the ethyl acetate fraction showed moderate hepatotoxicity at the maximal dose (200 μg/ml). This in vitro observation, therefore, offers a cautionary warning on the high consumption of yerba mate in vivo. Further, I. paraguariensis extract subjected to HPLC analysis led to identification of known anti-HBV active flavonoids, i.e. rutin, quercetin, kaempferol, caffeic acid and chlorogenic acid, in line with previous studies [12–15, 33].
In addition to the health-protective or therapeutic bioactive natural products commonly consumed as dietary supplements, notably flavonoids, to compensate nutritional deficiencies [34], physical exercise and weight control are widely recommended to rejuvenate and recover compromised liver health [35, 36]. Of the known bioactive phytochemical classes, flavonoids and polyphenols constitute the largest source of promising antiviral compounds against several viruses, including HBV [6, 37–39]. The flavonoids rutin, quercetin, myricetin and kaempferol as well as some of their derivatives have been recently demonstrated to have strong anti-HBV activity in HepG2.2.15 cells [10, 12–15]. The polyphenol caffeic acid has been widely reported for its strong activity against herpes simplex virus, influenza virus, poliovirus, and hepatitis C virus, vaccinia virus and severe fever with thrombocytopenia syndrome virus [40–43]. Its derivative, chlorogenic acid, has been also shown to have broad-spectrum antiviral activity against HIV [44], adenovirus [45], and HSV [46, 47]. However, the anti-HBV activity of caffeic acid or chlorogenic acid remains underreported. In a single published study, they are demonstrated to effectively suppress HBV antigens and DNA in HepG2.2.15 as well as in duck HBV (DHBV) models [33].
In HPLC validation, the noncytotoxic preparations of I. paraguariensis were tested for their anti-HBV activity in HepG2.2.15. Notably, the HepG2.2.15 line comprises stably-transfected HepG2 cells with the HBV genome that allows its DNA replication and gene (surface, polymerase, capsid, pre-core, etc.) expression, and therefore is universally used to test anti-HBV agents. Of the tested noncytotoxic preparations (50 μg/ml), the chloroform fraction showed moderate (40–42.3%) whereas hexane and ethanol fractions exhibited high (52–55.6%) anti-HBV activity. Very convincingly therefore, our data on in vitro anti-HBV activity of I. paraguariensis could be attributed to rutin, quercetin, kaempferol, caffeic acid and chlorogenic acid, as validated by HPLC. Furthermore, based on our in silico molecular docking analysis, we have previously reported evidence for antiviral activity of rutin, quercetin and kaempferol through binding with HBV polymerase and capsid proteins [12, 15]. In the present study, we have further documented the interactions of caffeic acid and chlorogenic acid with HBV polymerase with good docking affinities.
In conclusion, the in vitro anti-HBV activity of I. paraguariensis is strongly supported by HPLC validation and molecular docking. In conclusion, we, for the first time, demonstrated the HBV inhibitory activity of I. paraguariensis attributed to its bioactive phytochemicals rutin, quercetin, kaempferol, caffeic acid and chlorogenic acid in a HBV-reporter cell culture model, validated by high performance liquid chromatography and molecular docking analysis. Our findings, therefore, warrant further experimental and pharmacological studies on I. paraguariensis.