Introduction
Polyphenols are secondary plant metabolites and bioactive compounds naturally occurring in plants, plant-derived products, and fungi [1].
During the last decades, the potential beneficial effects of flavonoids, especially on human cardiovascular health, has caught the attention of researchers across the world. Indeed, flavonoids are able to decrease the oxidation of low-density lipoproteins. Furthermore, flavonoids positively impact the cardiovascular system, thanks to their ability to produce vasodilation and regulate apoptotic processes in the endothelium. It seems that the great antioxidant properties of flavonoids drive all these effects, along with their anti-inflammatory function; however, recently, different mechanisms involved in the beneficial effect of flavonoids on the human body have been identified, showing multiple signalling pathways related to them [2]. Some of them have also been associated with improvement of symptoms in heart failure patients [3].
Pooling data from several epidemiological and clinical studies, total flavonoids and specific subclasses have been associated with a reduced incidence of cardiovascular diseases (CVD), diabetes mellitus, and all-cause mortality [4].
Flavonoids have been shown to act as free radical scavengers and exert antioxidant, hepatoprotective, and anti-inflammatory activities [5]. Flavonoids’ biological activities reflect their chemical and biochemical properties, including the ability to regulate the gene expression in chronic diseases and modulate several molecular pathways [6].
Recently, bergamot and opuntia standardized extracts have been suggested as safe natural compounds that are able to modify some components of the metabolic syndrome [7, 8]. In particular, Citrus bergamia Risso, a citrus fruit native to southern Italy, known as bergamot, with uses including the improvement of immune response and cardiovascular function, has shown a significant degree of hypercholesterolaemic and antioxidant activity. It also has a particular flavonoid composition, as it contains some flavanones that may act as natural statins. Multiple clinical studies have provided evidence that different forms of orally administered bergamot can reduce total cholesterol and low-density lipoprotein cholesterol [9, 10].
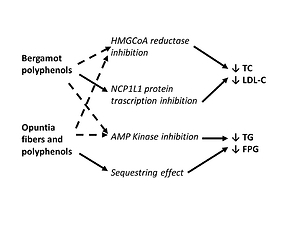
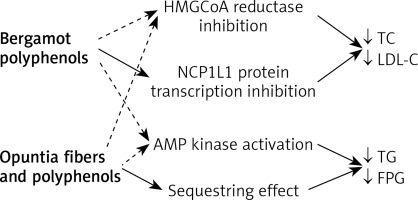
At the same time, over the past decade, academic scientists and private companies have provided compelling evidence of the potential of Opuntia ficus Indica. Due to its rich composition in polyphenols, vitamins, polyunsaturated fatty acids, and amino acids it has been shown to possess anti-inflammatory, antioxidant, hypoglycaemic, antimicrobial, and neuroprotective properties [11]. The hypoglycaemic properties seem to be related to the fibrous component (soluble fibres), such as pectins and mucilages, which exert a mechanical effect on the binding of sugars taken with the diet, thus reducing their absorption, and increasing the faecal excretion. Its cholesterol-lowering properties may be ascribed to a metabolic action, linked to the cholesterol pathways (Figure 1). This can be exerted partly by the pectin component and partly to the polyphenolic component, also responsible for the antiatherogenic activity [10, 12].
Figure 1
Possible metabolic effect of bergamot and opuntia polyphenols in humans
Continuous lines – main action, dotted line – minor effects, HMGoA – β-Hydroxy β-methylglutaryl-coenzyme A, NC1L1 – Niemann-Pick C1-like 1, AMP – 5’ adenosine monophosphate-activated protein.
In this context, we could suppose that a combined dietary supplement rich in polyphenols and fibres derived from the Mediterranean fruits could have a global positive impact on human metabolism. Then, we aimed to evaluate if dietary supplementation with a nutraceutical compound containing bergamot and opuntia extracts positively affects serum lipid concentrations, glucose metabolism, and liver parameters in healthy hypercholesterolaemic subjects with low estimated cardiovascular risk.
Material and methods
We carried out a 3-parallel-arm, double-blind, placebo-controlled, randomized clinical trial in 75 hypercholesterolaemic subjects in primary prevention and low estimated risk for cardiovascular disease.
The study was conducted in accordance with the Declaration of Helsinki, its protocol was approved by the Ethical Committee of the University of Bologna, and informed consent was obtained from all patients before their inclusion in the study. Inclusion criteria were age between 18 and 70 years and low-density lipoprotein-cholesterol (LDL-C) level between 115 and 190 mg/dl, confirmed in at least 2 sequential checks before signing the consent form.
Enrolled patients were age and sex matched and were consecutively enrolled in the outpatient service of cardiovascular disease prevention of the Medical and Surgical Sciences Department of the University of Bologna.
Exclusion criteria were as follows:
– Personal history of cardiovascular disease or risk equivalents,
– Smoking habit,
– Triglycerides (TG) > 400 mg/dl and/or high-density lipoprotein-cholesterol (HDL-C) < 35 mg/dl,
– Obesity (body mass index (BMI) > 30 kg/m2),
– Assumption of lipid-lowering drugs or drugs affecting lipid metabolism,
– Known thyroid, liver, renal, or muscle diseases.
At baseline, patients were given standard behavioural and qualitative (not quantitative) dietary suggestions to correct unhealthy habits. Standard diet advice was given by a dietitian and/or specialist doctor, who periodically provided instruction on dietary intake, recording the procedures as part of a behaviour modification program, and then later used the subject’s food diaries for counselling. In particular, subjects were instructed to follow the general indication of a Mediterranean diet, avoiding excessive intake of dairy products and red meat-derived products during the study, maintaining overall constant dietary habits. Individuals were also encouraged to increase their physical activity by walking briskly for 20 to 30 min, 3 to 5 times per week, or by cycling.
Treatments
After 2 weeks of diet and physical activity, the patients were allocated to treatment with indistinguishable tablets containing placebo or low-dose active or high-dose active, in 3 groups defined as follows:
AA (25 subjects were given 2 A boxes), AB (25 subjects were given one A and one B box), BB (25 subjects were given 2 B boxes). A contained a nutraceutical composition (Verum) of 150 mg of Opuntia ficus Indica extract (75% pectins and mucilages, 3.7% polyphenols), 400 mg of Plant sterols, 12.5 mg of Thiamine, and 200 mg of Brumex®, a phytocomplex from bergamot fruit (Citrus bergamia Risso et Poiteau, fructus) standardized min 40% total flavonoids and min 5% in HMG (3-hydroxy-3-methylglutaryl-) flavanones. B contained a placebo (microcrystalline cellulose, magnesium stearate, silicon dioxide, yellow iron oxide dye, chlorophyllin dye, coating agents).
Each subject was instructed to take one tablet from each box assigned in the evening, before sleeping.
The treatment was then continued for 12 weeks. Clinical and laboratory data were obtained at the baseline and at the end of the trial. Randomization was done using a drawing of envelopes containing randomization codes prepared by an independent statistician using specific software. The envelopes were then further mixed and distributed to the investigators, who assigned the randomization code in a progressive way to the enrolled subjects. A copy of the code was provided only to the person responsible for performing the statistical analysis.
Patients were advised to take the first dose on the day after they were given the study product in a blinded box. At the same time, all unused products were retrieved for inventory. Product compliance was assessed by counting the number of product doses returned at the time of specified clinic visits.
Assessments
Body weight, waist circumference, and blood pressure were measured at each visit.
All plasma parameters were obtained after a 12-hour overnight fasting. Venous blood samples were drawn by a nurse from all patients between 8:00 a.m. and 9:00 a.m. Plasma used was obtained by addition of Na2EDTA (1 ml) and centrifuged at 3000 g for 15 min at 48°C. Immediately after centrifugation plasma samples were frozen and stored at –80°C for no more than 3 months. The following parameters were evaluated via standardized methods: [13, 14] total cholesterol (TC), HDL-C, TG, LDL-C, apolipoprotein AI (apoAI), apolipoprotein B100 (apoB), fasting plasma glucose (FPG), glutamate oxaloacetate transaminase (GOT), glutamate pyruvate transaminase (GPT), liver transaminases, γ-glutamyl-transferase (gGT), and creatine-phospho-kinase (CPK). All measurements were performed by trained personnel in the Lipid Clinic laboratory of the Medicine and Surgery Sciences Department.
Statistical analysis
Data were analysed using intention to treat by means of the Statistical Package for Social Sciences (SPSS) 25.0 for Windows. The sample size suggested to detect a mean difference of 5% between treatments in term of LDL-reduction, with a power of 0.90 and an α error of 0.05, was of at least 20 subjects per group. As per the protocol, we decided a priori to check the efficacy of treatments in subjects assuming at least the 90% of the tested products doses foreseen by the trial design. Normally distributed baseline characteristics of the population were compared using Student’s t test and the χ2 test followed by Fisher’s exact test for categorical variables. Between-group difference was assessed by ANOVA followed by Tukey’s post-hoc test. Inferential analyses were exploratively repeated by gender. All data are expressed as means and SD. A p level < 0.05 was considered significant for all tests.
Results
The baseline characteristics of patients assigned to the different treatment groups (25 per group) were similar, and no significant differences were observed regarding the studied parameters (Table I and II).
Table I
Age, anthropometric, and haemodynamic characteristics of the enrolled subjects under different treatment regimens at different study timepoints
| Parameter | Pre-run-in | Baseline | T1 | ||||
|---|---|---|---|---|---|---|---|
| Placebo | Verum low dose | Verum full dose | Placebo | Verum low dose | Verum full dose | ||
| Age [years] | 52 ±5 | 54 ±4 | 56 ±3 | 53 ±5 | 52 ±5 | 54 ±4 | 56 ±3 |
| BW [kg] | 63 ±4 | 64 ±5 | 65 ±3 | 63 ±4 | 64 ±5 | 64 ±4 | 63 ±3* |
| WC [cm] | 88 ±6 | 90.4 ±4.4 | 92.9 ±4.7 | 88.0 ±6.1 | 89.1 ±4.7 | 90.8 ±4.1 | 86.4 ±6.5 |
| BMI [kg/m2] | 22.8 ±2.2 | 23.7 ±1.7 | 24.7 ±1.9 | 22.8 ±2.2 | 23.6 ±1.5 | 23.7 ±2.4 | 21.3 ±2.3* |
| SBP [mm Hg] | 134 ±5 | 136 ±5 | 135 ±4 | 134 ±5 | 135 ±4 | 135 ±4 | 136 ±4 |
| DBP [mm Hg] | 87 ±2 | 88 ±3 | 86 ±3 | 87 ±3 | 87 ±2 | 85 ±3 | 87 ±2 |
Table II
Effect of different nutraceutical regimens on investigated laboratory parameters
| Parameter | Pre-run-in | Baseline | T1 | ||||
|---|---|---|---|---|---|---|---|
| Placebo | Verum low dose | Verum full dose | Placebo | Verum low dose | Verum full dose | ||
| TC [mg/dl] | 248 ±13.0 | 239.8 ±11.7 | 246 ±7 | 238 ±13 | 241 ±13 | 210 ±12*° | 205 ±13*° |
| HDL-C [mg/dl] | 44 ±3 | 46.5 ±3 | 46 ±2 | 44 ±3 | 44 ±3 | 49 ±1*° | 50 ±2*° |
| LDL-C [mg/dl] | 161 ±8 | 150.7 ±10 | 159 ±6 | 155 ±8 | 157 ±9 | 127 ±9*° | 125 ±8*° |
| Non-HDL-C [mg/dl] | 204 ±11 | 197.4 ±10 | 207 ±8 | 198 ±11 | 197 ±12 | 161 ±11*° | 155 ±12*° |
| TG [mg/dl] | 216 ±19 | 205.4 ±14 | 195 ±23 | 197 ±16 | 198 ±18* | 170 ±16*° | 156 ±12*°# |
| ApoB [mg/dl] | 146 ±9 | 140.1 ±8 | 143 ±7 | 143 ±7 | 141 ±7 | 120 ±9*° | 119 ±9*° |
| ApoAI [mg/dl] | 118 ±12 | 101.9 ±12 | 113 ±14 | 114 ±16 | 118 ±14 | 137 ±13*° | 139 ±11*° |
| FPG [mg/dl] | 88 ±3 | 89.5 ±3 | 89 ±3 | 90 ±3 | 88 ±3 | 84 ±2* | 83 ±4* |
| GOT [U/l] | 23 ±3 | 24.5 ±3 | 24 ±2 | 25 ±4 | 25 ±3 | 22 ±2* | 21 ±3* |
| GPT [U/l] | 22 ±3 | 21.9 ±2 | 22 ±2 | 22 ±3 | 22 ±3 | 20 ±4* | 20 ±4* |
| gGT [mg/dl] | 32 ±2 | 34.6 ±2 | 37 ±2 | 33 ±2 | 33 ±2 | 24 ±3* | 23 ±4* |
| CPK [U/ml] | 104 ±19 | 101.3 ±21 | 118 ±19 | 95 ±15 | 120 ±25 | 106.8 ±24.4 | 96.1 ±18.6 |
# p < 0.05 vs. placebo and verum low-dose. SBP – systolic blood pressure, DBP – diastolic blood pressure, TC – total cholesterol, LDL-C – low-density lipoprotein cholesterol, HDL-C – high-density lipoprotein cholesterol, TG – triglycerides, Apo – apolipoprotein, FPG – fasting plasma glucose, GOT – glutamic-oxaloacetic transaminase, GPT – glutamate-pyruvate transaminase, gGT – γ-glutamyl transferase, CPK – creatine phosphokinase.
Men and women were equally distributed among groups: 13 : 12 (placebo), 12:13 (low-dose verum), 11 : 14 (full-dose verum).
Body weight and BMI significantly decreased versus baseline in the full-dose nutraceutical-treated group (p < 0.05). No significant change was detected during the study in all 3 treatment groups as regards blood pressure (for all, p > 0.05) (Table I).
From the randomization visit to the end of the study, the enrolled subjects maintained overall a similar dietary pattern, without significant change in total energy, TC, and total saturated fatty acid intake. We observed that the low dose of the tested product significantly reduced a number of metabolic parameters versus baseline: TC = –14.6%, LDL-C = –19.9%, non-HDL-C = –22.1%, TG = –13.1%, ApoB = –16% (all p < 0.05 both versus baseline and versus placebo), FPG = –5.1%, GOT = –7.8, GPT = –7.3%, and gGT = –34.4% (all, p < 0.05 versus baseline). HDL was increased 6.9% by the use of one tablet per day (p < 0.05 versus baseline). All parameters were reduced at the same extent when taking the full dose (2 tablets). However, the full dose showed a better impact on TG (–20.8%) and HDL (13.6%) (all, p < 0.05 versus baseline) (Table II).
The placebo group experienced a significant reduction in TG versus baseline, only (p < 0.05), as a marker of attention to the suggested diet (Table II).
The group treated with a single daily dose of the tested nutraceutical compound experienced a significant improvement in the level of all the lipid fractions and lipoproteins levels compared to placebo (p < 0.05), but not of liver parameters (p > 0.05) (Table II).
The exploratory repetition of the inferential statistics by gender did not show any significant difference in the observed metabolic changes in men and women.
Discussion
A healthy lifestyle remains the cornerstone of cardiometabolic disease prevention [15]. However, during the last decades great attention has been paid to nutraceutical compounds able to improve the LDL-C [7, 16] level and metabolic syndrome components [6, 17]. Among them, polyphenols are of particular interest for their pleiotropic effect on metabolism and vascular health [18]. However, further clinical data are always required to find the most effective products.
In our double-bind, placebo-controlled clinical trial, the groups treated with either a single or a double dose of the tested nutraceutical compound experienced a significant decrease in TC, LDL-C, non-HDL-C, TG, ApoB, FPG, GOT, GPT, and gGT, while a significant increase of HDL-C and ApoAI plasma level was seen after 12 weeks of treatment. The results are in agreement with previous literature showing that, in humans, the extract derived from bergamot exerts positive effects on hyperlipidaemia, demonstrating an effect in the modulation of total cholesterol, triglycerides, and LDL [19]. In particular, bergamot polyphenolic fraction has been demonstrated to improve lipid metabolism by different mechanisms of action: inhibition of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase (naringin, brutieridin, and melitidin), modulation of expression and activity of acyl-CoA oxidase, stearoyl-CoA desaturase 1, and liver-fatty acid binding protein (flavolignans), inhibition of acyl CoA:cholesterol O-acyltransferase (naringin, hesperidin), binding of biliary salts, and improving faecal excretion of sterols [20]. However, the results observed in the current study might be due to the concomitant action of bergamot flavonoids and opuntia pectins and mucilages on the lipid levels. Opuntia mucilages and pecting could also improve glucose and lipid pattern, by slowing and partly inhibiting the absorption of food carbohydrates, sterols, and lipids in the bowel [21]. Moreover, we already showed that opuntia was able to improve plasma lipid level and atheromasic LDL subfractions in overall healthy subjects [22] and in individuals with metabolic syndrome and type 2 diabetes, with significant action in reducing TC and LDL [23]. Finally, phytosterols have been shown also to improve the excretion of cholesterol and the reduction of LDL, mainly competing with dietary and biliary cholesterol absorption in the bowel [24]. Supplementation of phytosterol can be of relevant importance in the case of diet with a low basal content of phytosterols, as in some cases in the Western diet. All together, these mechanisms of actions could justify the final effect of the tested dietary supplement on the plasma lipid level.
The observed improvement in FPG and liver enzyme was also expected. In particular, in a previous double-blind, randomized clinical trial carried out with the same bergamot extract tested in this study, we observed a significant improvement in plasma lipid levels, insulin-resistance, leptin, leptin/adiponectin ratio, high sensitivity-C reactive protein, and tumour necrosis factor-α plasma level [25]. Incidentally, the potential anti-inflammatory effect of some nutraceuticals and its implication of cardiovascular disease prevention has been recently highlighted by a large expert panel [26].
Given the need of long-term intake, nutraceutical safety is also of growing interest [27]. Overall, the mid-term tolerability components of the tested combined nutraceuticals have been largely confirmed by the available literature and by our trial. Longer duration studies are needed to further confirm this observation on the tested nutraceutical combination.
We must acknowledge some study limitations. In particular, the trial was short, so we could not infer that a longer intake of the tested dietary supplement would have led to eventual further improvement of the studied parameters or to a reduction of the observed positive effect. Moreover, the sample size of the study was relatively small, even if adequately powered for the aim of the trial. For the same reason, no advanced statistics was carried out (namely regression to individuate predictors of better or worse answer to the tested product). As a consequence, further long-term data on a larger patient sample should be obtained in a new, double-blind, randomized clinical trial in order to confirm (or refute) our current observations.
However, based on our preliminary data, we suggest that synergy between Citrus bergamia polyphenols and Opuntia Ficus extracts could be an effective option to expand the therapeutic role in dyslipidaemic patients.
In general, the tested product combined with an improvement in life-style could be considered for the management of mildly dyslipidaemic subjects with low-added cardiovascular risk [28, 29]. The lack of statin-like compounds in the tested product will also support the hypothesis of its use in statin-intolerant patients at low-added cardiovascular risk, maybe in association with ezetimibe [30].
In conclusion, the tested nutraceutical compound based on a flavonoid complex from bergamot and opuntia showed a short-term positive impact on plasma lipids, fasting plasma glucose, and liver enzyme in overall healthy subjects affected by hypercholesterolaemia and low-added cardiovascular disease risk. Further long-term studies are expected to confirm the metabolic benefit of bergamot polyphenols in combination with Opuntia Ficus indica extracts.