Introduction
Hypertension is a major health and economic burden around the world due to its increasing prevalence and incidence [1–4]. In addition, hypertension contributes to the development and progression of chronic heart failure (CHF), coronary heart disease (CHD) and ischemic or hemorrhage stroke [5–8]. Numerous randomized controlled trials and meta-analyses have consistently demonstrated that reducing blood pressure (BP) with medications is beneficial for primary and secondary prevention of composite cardiovascular diseases (CVD) [9–11].
Observational studies have shown that compared to patients without hypertension, hypertensive patients commonly have increased arterial stiffness. In addition, hypertensive patients with increased arterial stiffness are less likely to have BP control compared to their counterparts without increased arterial stiffness [12–14]; and increased arterial stiffness in hypertensive patients is associated with higher prevalence and incidence of target organ damage and composite CVD [13, 15, 16]. However, no effective and efficient treatment is currently available to ameliorate arterial stiffness. Although some post-hoc analyses from prior clinical trials have shown that specific antihypertensive medication such as calcium channel blocker was associated with lower arterial stiffness and cardiovascular events versus β-blocker [17], no randomized controlled trials had ever been done to evaluate whether improvement of arterial stiffness can prevent cardiovascular events in these populations. In addition, the data on the Chinese population are scarce.
Notably, the aldosterone receptor antagonist spironolactone is an antihypertensive medication and is characterized by its pleiotropic effects in terms of improving endothelial function, ameliorating oxidative stress and decreasing fibrosis accumulation [18, 19]. However, whether spironolactone treatment is beneficial for improvement of arterial stiffness is unknown. Therefore, in the current study, we used a cross-sectional design to evaluate the association of spironolactone treatment and arterial stiffness in hypertensive patients. In addition, whether spironolactone treatment is associated with lower prevalence of composite CVD in patients with arterial stiffness was also investigated.
Material and methods
Participants’ enrollment
The current study was conducted between January and October of 2018 and the protocol of the current study was approved by the Research Ethics Committee of FuWai Hospital Chinese Academic of Medical Science. All participants provided informed consent before enrollment. The inclusion criteria were as follows: documented primary hypertension and being able to complete arterial stiffness measurement. The exclusion criteria were as follows: documented secondary hypertension, pregnant woman, had acute heart failure, acute myocardial infarction, or ischemic or hemorrhage stroke in the last 6 months, or had end stage renal disease or maintained hemodialysis.
Baseline data collection
Demographics including age, gender and body mass index (BMI) calculated by weight in kilogram divided by height in squared meters; composite CVD risk factors including smoking status, dyslipidemia and diabetes mellitus; composite CVD including CHF, CHD, and ischemic stroke; and current medications used were collected by two independent investigators using a structured questionnaire.
Assessment of arterial stiffness
The assessment of arterial stiffness was performed based on prior report without slight modification [20]. Carotid-femoral pulse wave velocity (cf-PWV) was measured to evaluate arterial stiffness using applanation tonometry (SphygmoCor; AtCor Medical, Sydney, Australia). Specifically, the travel distance by the pulse wave over the surface of the body was measured with a tape measure from the sternal notch to the right carotid artery, and from the sternal notch to the right femoral artery. The time delay was recorded between the troughs of these two waveforms, and then the distance was divided by the transit time. Before arterial measurement, participants were required to be fasting and put in a supine position and three measurements were performed to obtain the median values in accordance with the guideline recommendation [21].
Laboratory data
Before arterial stiffness measurement, fasting venous blood was drawn for evaluation of serum levels of fasting plasma glucose (FPG), total cholesterol (TC), C-reactive protein and creatinine, which were measured using an automatic biochemistry analyzer. Serum creatinine level was used to calculate glomerular filtration rate (GFR) based on the MDRD formula [22]. Plasma aldosterone concentration (PAC) and plasma renin activity (PRA) were determined by radioimmunoassay and aldosterone/renin ratio (ARR) calculated by the standard formula of PAC divided by PRA was also recorded.
Statistical analysis
Continuous variables were presented as mean ± SD and categorical variables were presented by proportion and number. Between-group differences were analyzed by Student’s t-test for continuous variables and the χ2 or Fisher exact test for categorical variables. Logistic regression analysis was performed to evaluate the associations of spironolactone treatment and arterial stiffness; and the association of spironolactone treatment and prevalence of composite CVD (including CHF, CHD and ischemic stroke) in patients with and without increased arterial stiffness, respectively. Statistical analysis was computed using SPSS 17.0 (SPSS Inc, Chicago, IL). All statistical tests were two-sided and considered statistically significant when p < 0.05.
Results
Baseline characteristics comparisons
Patients were divided into two groups – with (n = 170) and without (n = 274) spironolactone treatment. As presented in Table I, compared to patients without spironolactone treatment, those with spironolactone treatment were older (53.8 ±17.7 vs. 50.4 ±16.2 years), more likely to be male (64.7% vs. 57.7%), current smokers (40.6% vs. 37.2%), had higher serum level of CRP (5.8 ±2.0 vs. 3.2 ±1.1 mg/dl), creatinine (73.4 ±21.5 vs. 68.4 ±20.2 μmol/l) but lower GFR (85.4 ±9.6 vs. 92.6 ±10.7 ml/min/1.73 m2), and more likely to have diabetes mellitus (34.1% vs. 29.9%), CHF (10.6% vs. 7.3%) and aspirin treatment (31.8% vs. 28.5%). In addition, patients with spironolactone treatment also had lower PAC (8.7 ±2.4 vs. 10.7 ±3.6 ng/dl) and ARR (6.0 ±1.5 vs. 7.6 ±1.8).
Table I
Comparison of baseline characteristics
| Variables | Without spironolactone (n = 274) | With spironolactone (n = 170) |
|---|---|---|
| Age [years] | 50.4 ±16.2 | 53.8 ±17.7* |
| Male, n (%) | 158 (57.7) | 110 (64.7)* |
| Current smoker, n (%) | 102 (37.2) | 69 (40.6)* |
| BMI [kg/m2] | 23.4 ±5.2 | 24.1 ±5.5 |
| FPG [mg/dl] | 96.4 ±12.7 | 97.3 ±13.1 |
| Total cholesterol [mmol/l] | 5.0 ±0.9 | 5.0 ±1.0 |
| CRP [mg/dl] | 3.2 ±1.1 | 5.8 ±2.0* |
| Creatinine [μmol/l] | 68.4 ±20.2 | 73.4 ±21.5* |
| eGFR [ml/min/1.73 m2] | 92.6 ±10.7 | 85.4 ±9.6* |
| Diabetes mellitus, n (%) | 82 (29.9) | 58 (34.1)* |
| Dyslipidemia, n (%) | 76 (27.7) | 46 (27.1) |
| CHF, n (%) | 20 (7.3) | 18 (10.6)* |
| CHD, n (%) | 22 (8.0) | 16 (9.4) |
| Ischemic stroke, n (%) | 19 (6.9) | 15 (8.8) |
| Aspirin, n (%) | 78 (28.5) | 54 (31.8)* |
| Statins, n (%) | 72 (26.3) | 48 (28.2) |
| Antidiabetics, n (%) | 78 (28.5) | 50 (29.4) |
| PAC [ng/dl] | 10.7 ±3.6 | 8.7 ±2.4* |
| PRA [ng/ml/h] | 4.2 ±2.0 | 4.4 ±2.2 |
| ARR | 7.6 ±1.8 | 6.0 ±1.5* |
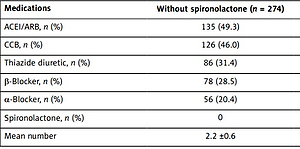
Comparisons of antihypertensive medications
Antihypertensive medications used at baseline were evaluated and compared between these two groups. As presented in Table II, no significant differences in antihypertensive medications used were observed except for spironolactone treatment. The mean number of antihypertensive medications used was significantly higher in the spironolactone group versus the treatment group without spironolactone (2.6 ±0.8 vs. 2.2 ±0.6).
Table II
Comparison of antihypertensive medications
| Medications | Without spironolactone (n = 274) | With spironolactone (n = 170) |
|---|---|---|
| ACEI/ARB, n (%) | 135 (49.3) | 83 (48.8) |
| CCB, n (%) | 126 (46.0) | 80 (47.1) |
| Thiazide diuretic, n (%) | 86 (31.4) | 55 (32.4) |
| β-Blocker, n (%) | 78 (28.5) | 50 (29.4) |
| α-Blocker, n (%) | 56 (20.4) | 35 (20.6) |
| Spironolactone, n (%) | 0 | 170 (100) |
| Mean number | 2.2 ±0.6 | 2.6 ±0.8* |
Comparisons of arterial stiffness
Arterial stiffness were compared and as presented in Table III, no significant differences in peripheral BP levels were observed. However, compared to patients without spironolactone treatment, those with spironolactone treatment had significantly lower cf-PWV (9.4 ±1.8 vs. 10.1 ±2.2 m/s).
Table III
Comparison of arterial stiffness
| Variables | Without spironolactone (n = 274) | With spironolactone (n = 170) |
|---|---|---|
| Peripheral systolic BP [mm Hg] | 122 ±18 | 124 ±19 |
| Peripheral diastolic BP [mm Hg] | 75 ±9 | 78 ±8 |
| Heart rate [beats/min] | 74 ±18 | 73 ±16 |
| Cf-PWV [m/s] | 10.1 ±2.2 | 9.4 ±1.8* |
Associations of spironolactone treatment and arterial stiffness
As shown in Table IV, in the unadjusted model, spironolactone treatment was associated with 20% lower risk of arterial stiffness. With stepwise regression models, after adjusting for potential confounding factors, spironolactone treatment was still associated with 10% lower risk of arterial stiffness, with a 95% confidence interval of 0.85–0.97.
Table IV
Associations of spironolactone treatment and arterial stiffness
| Independent variables | Odds ratio | 95% Confidence interval |
|---|---|---|
| Unadjusted* | 0.80 | 0.74–0.89 |
| Model 1* | 0.83 | 0.78–0.92 |
| Model 2* | 0.87 | 0.81–0.94 |
| Model 3* | 0.90 | 0.85–0.97 |
Model 1 – adjusted for age, male gender, body mass index; model 2 – further adjusted for smoking status, diabetes mellitus, C-reactive protein, glomerular filtration rate, chronic heart failure and peripheral systolic blood pressure; model 3 – further adjusted for plasma aldosterone concentration, calcium channel blocker, and β-blocker;
Associations of spironolactone treatment and prevalence of composite CVD
Based on the Expert Consensus recommended cutoff values of cf-PWV [23], patients were divided into groups without arterial stiffness (cf-PWV < 9.6 m/s) and with increased arterial stiffness (cf-PWV ≥ 9.6 m/s) and the associations of spironolactone treatment and prevalence of composite CVD were evaluated. As presented in Table V, in the group without arterial stiffness, after stepwise regression analysis, no significant association of spironolactone treatment and prevalence of composite CVD was observed. However, in the increased arterial stiffness group, after stepwise regression analysis, spironolactone treatment was still independently associated with 11% lower risk of composite CVD, with a 95% confidence interval of 0.83–0.97.
Table V
Associations of spironolactone treatment and prevalence of composite CVD
| Independent variables | Odds ratio | 95% Confidence interval |
|---|---|---|
| Without arterial stiffness (n = 262): | ||
| Unadjusted* | 0.87 | 0.79–0.92 |
| Model 1* | 0.90 | 0.85–0.98 |
| Model 2 | 0.94 | 0.89–1.06 |
| Model 3 | 0.99 | 0.93–1.12 |
| Increased arterial stiffness (n = 182) | ||
| Unadjusted* | 0.78 | 0.72–0.86 |
| Model 1* | 0.82 | 0.77–0.90 |
| Model 2* | 0.86 | 0.80–0.93 |
| Model 3* | 0.89 | 0.83–0.97 |
Model 1 – adjusted for age, male gender, body mass index; model 2 – further adjusted for smoking status, diabetes mellitus, C-reactive protein, total cholesterol, glomerular filtration rate and peripheral systolic blood pressure; model 3 – further adjusted for plasma aldosterone concentration and calcium channel blocker, and β-blocker; CVD – cardiovascular disease;
Discussion
To our knowledge, this is the first study to evaluate the association of spironolactone treatment and arterial stiffness and prevalence of CVD in the Chinese hypertensive populations. The major findings of our current study were as follows: 1) spironolactone treatment was associated with lower cf-PWV values; 2) spironolactone treatment was independently associated with lower risk of arterial stiffness; 3) spironolactone treatment was also independently associated with lower risk of prevalent composite CVD in hypertensive patients with increased arterial stiffness but not in patients without arterial stiffness.
Notably, hypertension is a major risk factor for CVD and good control of hypertension is beneficial for primary and secondary prevention of CVD. However, observational studies suggested that a substantial proportion of hypertensive patients are difficult to have their blood pressure controlled and one of the potential mechanisms is related to arterial stiffness. It is well known that elevated BP results in arterial stiffness through direct stress shear, endothelial dysfunction and reduced nitric oxide production, oxidative stress and fibrosis accumulation in the vascular wall. These pathophysiological processes together in turn lead to arterial stiffness and further BP elevation. For example, AlGhatrif et al. [24] reported that a dose-dependent relationship between systolic BP elevation and PWV increase was observed. In addition, a large number of clinical studies have also indicated that increased cf-PWV value was associated with risk of CVD. For example, authors [15] reported that higher aortic stiffness assessed by PWV was associated with increased risk for a first cardiovascular event. Aortic PWV improved risk prediction when added to standard risk factors. In end stage renal dysfunction, Blacher et al. [25] also observed that a 1 m/s increase in aortic PWV was associated with a 39% higher risk of all-cause mortality. However, to date, no randomized controlled trials have shown whether improvement of arterial stiffness is beneficial for hypertension control and CVD reduction.
One observational study indicated that compared to β-blocker, calcium channel blocker was better for improvement of arterial stiffness as measured by aortic BP, which in turn led to fewer cardiovascular events [17]. In another study, Liu et al. [26] reported that compared to hydrochlorothiazide, spironolactone was superior to BP control and the potential mechanism was due to improvement of arterial stiffness. In our current study, we found that although the peripheral BP was similar between groups with and without spironolactone treatment, cf-PWV was significantly lower in patients with versus without spironolactone treatment. In addition, in the regression models, in order to reduce potential confounding factors, we adjusted for factors related to arterial stiffness and the result implied that spironolactone treatment was still independently associated with lower cf-PWV. These findings also suggested that spironolactone treatment was beneficial for arterial stiffness improvement. Future randomized controlled trials are needed to corroborate our current findings. Compared to prior reports, some novelties of our current study need to be mentioned: first of all, this is the first study to evaluate the influence of spironolactone on arterial stiffness and CVD in Chinese hypertensive populations. Findings from our current analysis provide preliminary evidence for future interventional studies. Second, our study indicated that spironolactone treatment is associated with lower arterial stiffness, which in turn might be beneficial for reduced prevalence of CVD, which has not been fully evaluated previously. Third, our current study also provided the serum aldosterone level and renin activity so as to evaluate the potential mechanisms related to the efficacy of spironolactone for arterial stiffness. Fourth, findings from our study suggested that the cardiovascular benefits of spironolactone for hypertensive patients might be limited to those with arterial stiffness, which has also not been elucidated before.
We further evaluated whether spironolactone treatment was associated with lower risk of CVD. Interestingly and importantly, as presented in Table V, we found that spironolactone treatment was associated with lower risk of CVD only in patients with increased arterial stiffness but not in patients without arterial stiffness. These findings indirectly implied that the benefits of spironolactone treatment for CVD might be due to its efficacy in arterial stiffness improvement. Indeed, prior studies indicate that patients with increased arterial stiffness had higher risk of CVD versus those without arterial stiffness [13, 27, 28]. Spironolactone treatment could improve endothelial function, inflammatory reaction and oxidative stress in the vascular wall [18, 29–31], which in turn may reduce cardiovascular events. Future randomized controlled trials are needed to evaluate whether spironolactone treatment is beneficial for reducing cardiovascular events in patients with increased arterial stiffness. The clinical relevance of our current findings are: first, the findings of our study further support the notion that arterial stiffness is a potential risk factor for hypertensive patients; and incorporation of arterial stiffness evaluation may help to predict CVD risk in the future; second, in hypertensive patients with arterial stiffness, physicians may select spironolactone as a preferred medication due to the potential cardiovascular benefits of spironolactone for patients with arterial stiffness; third, the current study provided more evidence to support the conduction of randomized clinical trials to prospectively test the benefits of spironolactone for hypertensive patients with arterial stiffness, which in turn can help to change the guideline recommendations in the future.
There are some limitations of our current study: first, this is a cross-sectional study and the inherent biases of the study design do not allow us to infer causal relationships. However, our current study provides insight into the association of spironolactone treatment and arterial stiffness and CVD. Second, although we adjusted for confounding factors, it is still possible that undetected and unmeasured covariates influenced our findings. Third, the current study was conducted in Chinese hypertensive patients, so future studies from other racial/ethnic groups are needed to corroborate our findings.
In conclusion, our current study shows that spironolactone treatment is independently associated with lower cf-PWV and lower prevalence of composite CVD in patients with increased arterial stiffness. Future randomized controlled trials are warranted to confirm whether spironolactone treatment is beneficial for prevention of cardiovascular events in hypertensive patients through improvement of arterial stiffness.