Introduction
Acute myeloid leukemia (AML) accounts for one-fourth of all the cases of adults leukemia in Western countries, with an incidence of about 4 cases per 100,000 citizens per year [1]. Median age at diagnosis is 69 years [1, 2]. Acute myeloid leukemia is a very heterogeneous disorder due to many pathways of leukemogenesis, and additional genetic abnormalities that appear in AML cells [2].
According to the Nomenclature Committee on Cell Death (NCCD), classification of cell death is divided into eight subtypes: apoptosis, autophagy, mitotic catastrophe, paraptosis, necrosis, oncosis, pyroptosis and anoikis [3, 4]. All these types of cell degradation can be consider as a potential target in the treatment of malignancies. In fact, apoptosis and autophagy are the most known processes of cell death, which is connected with development of novel, targeted treatment options in neoplastic disorders [3, 5]. Apoptosis disturbance in AML is a well-established phenomenon, which supports blast cells in their escape from programmed death [6–8]. One of the ways is overexpression of the inhibitor of apoptosis proteins (IAP) family. The IAP’s family is one of the most important groups of apoptosis-regulating proteins that inhibit crucial for cell death activation of caspase-3, -7 and -9 [9–11]. Eight human IAP family members have been identified: XIAP (X-linked inhibitor of apoptosis), cIAP-1 (cellular inhibitor of apoptosis 1), cIAP2 (cellular inhibitor of apoptosis 2), survivin, NAIP (neuronal apoptosis inhibitory protein), livin, BRUCE/Apollon and IAP-like protein 2 (inhibitor apoptosis protein-like protein 2) [11, 12]. Expression of the whole IAP family is regulated by a nuclear factor kappa B (NF-κB). The NF-κB constitutive activation is a common phenomenon in different cancers, and results in the aberrant expression of NF-κB target genes and leads to malignant transformation, metastatic dissemination, abnormal cell proliferation or resistance to cell death [12, 13]. Inhibitor of apoptosis proteins family can be inhibited by Smac/DIABLO (second mitochondrial-derived activator of caspases/direct IAP binding protein with low PI), Omi/HrtA2 (HTRA serine peptidase 2) and XAF-1 (XIAP-associated factor 1) [7–10]. Smac/DIABLO protein appears to be one of the strongest IAPs inhibitors [10–13].
Influence of separate IAP’s family members on the treatment outcome and survival in AML patients has been presented by different groups [14–17]. It has been shown that overexpression of IAP family members in AML leukemic blasts/cells correlates with a low complete response (CR) rate and shorter overall survival (OS) [14–16]. Especially, the high survivin protein expression is associated with shorter OS [18]. Studies on XIAP, have shown that its overexpression was associated with a lower CR rate and the worst OS as compared to the patients with none or low XIAP expression [15, 16]. In AML patients various expression of different IAP’s family members has been described. One common link of IAP’s family may be their transcription factor, i.e. NF-κB. Inhibition of NF-κB plays a key role in promoting apoptosis after treatment. There is only little data evaluating expression of NF-κB protein in AML patients. However, important role of Smac/DIABLO protein, the strongest IAP’s family inhibitor in AML, has been established [17]. A correlation between high Smac/DIABLO expression and a good treatment outcome has been observed [17].
Based on these experiences, we decided to study expression of IAP family regulator NF-κB protein and its influence on CR achievement, and survival in AML patients with simultaneous examination of the main IAP’s inhibitor Smac/DIABLO protein.
Material and methods
Patients
The study involved 109 patients with newly diagnosed AML and hospitalized between January 2008 and December 2012 in the Department of Hematology, Medical University of Lodz. Characteristics of the patients are summarized in Table I. The study was approved by the Research Ethics Committee of Medical University of Lodz. A written informed consent was obtained from all the patients.
Table I
Patients’ characteristics
[i] WBC – white blood cells, LDH – lactate dehydrogenase, WHO – World Health Organization, AML – acute myeloid leukemia, NOS – not otherwise specified, SWOG – Southwest Oncology Group, DA – daunorubicine plus cytarabine, DAC – daunorubicine plus cladribine, LD-AraC – low dose cytarabine, BSC – best supportive care.
The diagnosis was based on the Word Health Organization Classification of Haematopoietic and Lymphoid Tissues (WHO) [2]. Median age among the patients was 61 years (range from 18 to 87 years).
Forty-nine patients received an intensive induction chemotherapy according to the Polish Adult Leukemia Group (PALG) protocols, described in former PALG’s publications [19–21]. Fourteen and thirty-five patients received DA (daunorubicin and cytarabine) and DAC (daunorubicine, cytarabine and cladribine) induction treatment, respectively. Fifty-nine patients received non-intensive treatment, including 23 patients treated with low dose cytarabine (LD-AraC), 8 with azacytadine (AZA) and 22 with hydroxyurea (HU). Six patients underwent best supportive care (BSC) only. Ten patients underwent bone marrow transplantation procedure. Eight patients received allogeneic stem cell transplantation (SCT) (5 from unrelated and 3 from a sibling donor) and 2 patients had autologous SCT.
Response to the treatment was defined in accordance with the revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, treatment Outcomes and Reporting Standards for therapeutic Trials in Acute Myeloid Leukemia [1, 22]. Briefly, CR was defined as the presence of less than 5% of bone marrow (BM) blasts with a neutrophil count higher than 1.0 G/l, a platelet count higher than 100 G/l and no extramedullary disease. Partial remission (PR) was established with either 5–25% BM blasts, a 50% or a higher decrease in BM blasts, or BM blasts < 5% but with the presence of Auer rods. No response (NR) was established for the patients who did not fulfill any of the mentioned above criteria. Early death (ED) was defined as a death from any cause within 4 weeks after the induction therapy and was considered as the treatment failure. Disease free survival (DFS) was calculated from the first day of CR until documentation of a relapse [1]. Overall survival (OS) was calculated from the time of diagnosis until death or date of stem cell transplantation [1].
Laboratory tests
Blood and bone marrow sampling
Venous blood or bone marrow samples were collected at the time of diagnosis into pyrogen-free ethylenediaminetetraacetic acid (EDTA) tubes. Immunophenotyping of leukemic cells was performed routinely in the whole peripheral blood or bone marrow using the flow cytometry: the “lysed-not washed” method. A routine panel of monoclonal antibodies HLA-DR, CD34, CD45, CD13, CD15, CD33, CD117 conjugated with fluorescein isothiocyanate (FITC) and phycoerythrin (PE) was applied (all BD Pharmingen, San Diego, CA, USA).
Assessment of expression of NF-κB and Smac/DIABLO protein
Flow cytometry cell preparation
Peripheral blood or bone marrow mononuclear cells were isolated from EDTA samples by centrifugation on a Histopaque-1077 (Sigma Diagnostic, St Louis, MO, USA) density gradient, for 30 min at 3600 rpm. The interphase region containing mononuclear cells was collected and washed, once in Hanks’ Balanced Salt Solution (Biomed, Lublin, Poland) and, twice in phosphate buffered saline (PBS; Sigma Aldrich Chemie Gmbh, Steinheim, Germany). Then the samples were fixed in 1% paraformaldehyde (PFA) (15 min at 4oC) and in 90% ethanol for 30 min at –20oC for the flow cytometry analysis.
The frozen cells were washed in PBS, centrifuged (5 min, 1100 rpm) and incubated in 0.01% saponin for 1 min. The cells were then washed in PBS and centrifuged (5 min, 1100 rpm). The incubation with primary antibody (Ab) at a dilution of 1 : 50 of anti-NFκB (MAB28881, monoclonal mouse anti-human Ab, R&D System, Minneapolis, MN, USA) and 1 : 100 anti-Smac/DIABLO (AF 7891), polyclonal goat Ab, R&D System, Minneapolis, MN, USA) was performed at 4oC, overnight. On the following day, the samples were washed in PBS, centrifuged (5 min, 1100 rpm) and incubated for 120 min with secondary FITC-conjugated Abs at a dilution of 1 : 20. Afterwards, the samples were washed in PBS, centrifuged (5 min, 1100 rpm) before being resuspended in 400 µl PBS and subjected to the flow cytometry analysis. At the same time, samples with isotype controls were also prepared (Normal goat IgG control, 1 : 100 dilution, R&D System, Minneapolis, MN, USA; Normal mouse IgG control, 1 : 100 dilution, R&D System, Minneapolis, MN, USA). All preparations have been previously described in detail [14, 18].
Flow cytometry analysis
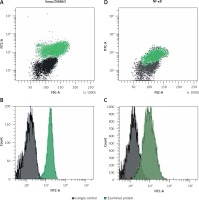
All fluorescence measurements were performed by the use of flow cytometry (FACScan; Becton-Dickinson, San Jose, CA, USA). An acquisition gate was established based on FSC (forward scatter) and SSC (side scatter) that included mononuclear cells according to the previous immunophenotype. Cell fluorescence was measured using standard emission filters: FL1 (green, λ 515–545 nm), FL2 (orange, λ 564–606 nm). For each analysis 10,000 events were acquired and analyzed using the CellQuestPro software (Becton Dickinson, San Jose, CA, USA). Expressions of Smac/DIABLO and NF-κB were presented as a percentage of Smac/DIABLO and NF-κB-positive cells in the whole population of leukemic blasts. All the flow cytometry measurements were performed on 10,000 cells per sample. The population of Smac/DIABLO and NF-κB-positive cells was identified after gating based on appropriate isotype controls. For data quantitation the FACS DIVA software was used. “High” and “low” expression was established according to the median of Smac/DIABLO and NF-κB-positive cells estimated in the whole group of patients. Examples of flow cytometry images are presented in Figure 1.
Statistical analysis
The statistical analysis was performed with the Statistica 12.0 (Tulusa, OK, USA) software. Statistical comparison of “low-expressers” and “high-expressers” was performed using the Mann-Whitney U test. Correlations between the variables were assessed using the Spearman rank test. Overall survival was estimated using the Kaplan-Meier method. The χ2 and logistic regression tests were applied to investigate dependence between individual factors and CR rate. The log-rank test was used to evaluate the univariate effects of particular variables on OS. For the multivariate analysis of factors affecting OS Cox, a proportional hazard regression model was applied. Comparisons and correlations between the examined parameters were considered significant when p < 0.05.
Results
Intracellular flow cytometry proteins’ expression
NF-κB and Smac/DIABLO protein expression was measured in 109 newly diagnosed AML patients. The expression of NF-κB and Smac/DIABLO protein was found in 104 of 109 (95%) and 107 of 109 (98%) patients, respectively. Median expression was 23.9% (0–94.7%) and 61.4% (ranged 0–99.9%) for NF-κB and Smac/DIABLO proteins, respectively.
Correlation between expression of NF-κB and Smac/DIABLO proteins in the acute myeloid leukemia patients
We evaluated the relationship between expression of NF-κB and Smac/DIABLO proteins. In the whole analyzed group a trend towards a negative correlation between the expression of Smac/DIABLO and NF-κB proteins was observed (R = –0.17, p = 0.06).
NF-κB and Smac/DIABLO expression and treatment outcome
Response to the treatment
The analyses of influence of NF-κB and Smac/DIABLO on treatment response were performed only in intensively treated patients’ group. Thirty-six (73%) of 49 intensively treated patients achieved CR. In the univariate analysis age below 60 years old, a good karyotype, the percentage of blast < 50% in bone marrow and higher expression of Smac/DIABLO were associated with a higher probability of CR achievement (p = 0.02, p = 0.007, p = 0.03, p = 0.03, respectively, Table II).
Table II
Univariate analysis (χ2 test) of factors associated with complete response rate in intensively treated patients
In the multivariate analysis, age below 60 years old and high Smac/DIABLO protein expression were the only factors that were associated with a higher probability of CR achievement (p = 0.008, and p = 0.04, respectively; Table III).
Overall survival
Median time of OS for the whole cohort of patients was 5 months (range: 0.03–81.6). The probability of 1-year survival was 32%. In the univariate log-rank analysis, better OS was influenced by age less than 60 years old, favorable or intermediate karyotype and higher expression of Smac/DIABLO (p < 0.0001, p < 0.039, p < 0.0015, respectively, Table IV). Additionally, the same factors: age < 60 years old, favorable or intermediate karyotype and high expression of Smac/DIABLO protein influenced longer OS in the multivariate Cox analysis (p = 0.00016, p = 0.01, p = 0.0014, respectively, Table V). In the survival univariate analysis of the subgroup treated with “apoptosis inducers” (standard induction or LD Ara-C) age below 60 years old and high Smac/DIABLO protein expression were found to be statistically significant risk factors for longer OS (p = 0.0005, p = 0.003, respectively) (Figure 2). Moreover, a trend towards better survival in the patients with lower NF-κB protein expression and less than 50% in bone marrow blasts was observed (p = 0.06 and p = 0.07, respectively) (Figure 2). In the multivariate survival analysis of this subgroup only age < 60 years old and high Smac/DIABLO protein expression was associated with longer OS (p = 0.0005 and p = 0.002, respectively).
Table IV
Univariate analysis (log rank test) of the factors associated with the probability of overall survival
Relationship between expression of NF-κB and Smac/DIABLO proteins and standard acute myeloid leukemia risk factors
Expression of NF-κB protein in the patients did not differ significantly in the risk groups divided according to the factors such as age (< 60 years vs. ≥ 60 years), karyotype (good, intermediate and unknown vs. poor), number of WBC count (≤ 20 G/l vs. < 20 G/l), percentage of leukemic blasts in bone marrow (≤ 50% vs. < 50%) and LDH (≤ UNL [upper normal limit] vs. < ULN). Similarly, expression of Smac/DIABLO protein did not correlate with all the mentioned above AML risk factors.
Discussion
Nuclear factor-κB plays an important role in survival of neoplastic cells also in AML. In AML patients NF-κB is constructively activated by different expression in each patient. This factor is crucial for activation of various mechanisms of escaping from cell death. One of those is stimulation of overproduction of IAP’s, which block apoptosis enzymes. In the presented study NF-κB and Smac/DIABLO protein expression was measured in the newly diagnosed AML patients’ blasts. Our study is the first one where simultaneously expression of NF-κB and expression of Smac/DIABLO proteins have been evaluated in the AML patients and correlated with the factors associated with clinical outcome of AML. Median expression was 23.9% and 61.4% for NF-κB and Smac/DIABLO proteins, respectively. A trend towards a negative correlation between the expression of NF-κB and Smac/DIABLO proteins was demonstrated.
The presence of NF-κB protein in U937 and HL-60 AML cell lines as well as patients’ blasts and leukemic stem cells has been previously described [23–29]. It has been shown that NF-κB is constitutively activated in AML blasts and leukemic stem cells of a large percent of patients [26]. In our cohort NF-κB expression was found in 104 of 109 (95%) patients, which is similar to other results [27]. NF-κB has been shown to upregulate transcription of proteins that promote cell survival, stimulate growth, induce angiogenesis and reduce sensibility to apoptosis. Expression of IAP’s family members has been shown to be regulated also by NF-κB [13]. Importantly, Schepers et al. have shown that constitutive activation of NF-κB plays a crucial role in survival of AML cells but not in the development of leukemia [30]. In our study, a trend towards shorter OS in the patients treated with “apoptosis inducers” schema with higher NF-κB expression was observed (p = 0.06). Lack of statistical significance was probably related to the not very big number of patients. These results are in accordance with Schepers’ observation that the patients with lower expression of NF-κB are more sensitive to chemotherapy, due to the lower activation of apoptosis escaping pathways [30].
Overexpression of natural IAP’s antagonist, Smac/DIABLO has been associated with a higher CR rate and better OS in AML patients in many studies [18, 31]. In the present study, high-expressers of Smac/DIABLO protein had longer OS. What is more, Smac/DIABLO overexpression was an independent risk factor of CR achievement in the multivariate analysis.
Smac/DIABLO is a small molecule, which induces apoptosis when it is released from the intramembrane space of mitochondrion to the cytosol. Many studies have evaluated the potential role Smac/DIABLO like proteins in cancer treatment. Recently, Safferthal et al. have shown that Smac/DIABLO mimetic circumvent apoptosis resistance in AML cell by inducing a programmed form of necrosis, necroptosis in a TNF-α dependent manner [32]. In vitro studies have shown that Smac/DIABLO like proteins decrease expression of IAP’s family members as well as NF-κB [33]. In our study a negative correlation between Smac/DIABLO and NF-κB beared out this observation.
Based on our observations, we may conclude that high NF-κB expression is an adverse risk factor of the treatment outcome. Moreover, high Smac/DIABLO protein expression is a good predictor of CR achievement and longer OS.
Further studies are necessary to establish the potential role of the combination of inhibitor of NF-κB with small molecules against IAP family, like Smac/DIABLO, in the treatment of AML patients.