Introduction
Cardiovascular disease (CVD) is a leading cause of death across Europe [1]. In line with European guidelines, patients with coronary heart disease (CHD) are considered to be at very high cardiovascular (CV) risk [2, 3].
In Central and Eastern European countries, higher rates of CHD mortality have been observed compared to other parts of this region [1]. Slovakia is no exception, with an annual incidence of 333.8 (males) and 209.5 (females) CHD-related deaths per 100,000 inhabitants [4]. Fortunately, CHD mortality is on the decline, though it is falling more slowly in Slovakia. In contrast to the 50–65% reductions in CHD-related death seen in Poland and the Czech Republic over recent years, only a 25% reduction has been observed in Slovakia [5–8].
An explanation for the poorer CHD statistics in Slovakia is the persistently high prevalence of chronic risk factors in the country’s population, including tobacco smoking (approximately 35% of the population) [9], high salt consumption [7], failure to meet the recommended levels of physical activity [10], and elevated serum cholesterol [11]. The majority of individual country analyses using the IMPACT model estimate that measures to reduce such risk factors account for 40–54% of the CHD-related mortality decrease observed over previous years [5, 6, 12–14]. This is in contrast to Slovakia, where most risk factors appear to be on the rise [7]. Overall, the studies agree that the reduction of serum cholesterol plays a particularly important role in reducing mortality, though Slovakia again presents an anomaly, with as many as 9% of the prevented or postponed deaths attributable to the use of lipid-lowering therapy (LLT) [7], compared to just 1.4–3.4% in the other countries [5–7, 12–14]. Considering these atypical trends and the observed slow decline in CHD-related mortality, data on the characteristics and management of CHD patients in Slovakia are of particular interest, though they are currently extremely limited.
Therefore, we designed a national observational registry to document real-world lipid levels of CHD patients in Slovakia and to provide insight into their accompanying characteristics, management strategies, and low-density lipoprotein cholesterol (LDL-C) target achievement.
Material and methods
The present study was an analysis of observational, prospective data on patients attending an outpatient visit for stable CHD at one of 35 sites across Slovakia (Bratislava: 12 sites, Nitra: 3 sites, Vrútky: 1 site, Martin: 1 site, Banská Bystrica: 6 sites, Prešov: 4 sites, and Košice: 8 sites) between November 2015 and January 2016. Sites were approached based on their regional distribution in Slovakia and recruitment relied on the physician’s willingness to participate. Consequently the data are potentially representative for the outpatient situation in Slovakia but do not constitute a picture of the situation in the general population. The registry protocol was an adapted version of that previously described for the DYSIS II international study [4, 15], an observational, cross-sectional, multicenter registry completed in 2014, which spanned Europe, Central and Eastern Africa, and the Asia/Pacific region [4, 15, 16]. The present study was approved by the relevant ethical review boards at the participating institutions and conducted in accordance with the Declaration of Helsinki and its amendments. Each patient provided written informed consent.
Inclusion/exclusion criteria
An average of 10 consecutive patients were enrolled at each participating center by the physician in charge. Inclusion criteria were as follows: age ≥ 18 years; CHD, defined as stenosis of > 50% at coronary angiography or cardiac computed tomography (CT), previous percutaneous coronary intervention (PCI), previous coronary artery bypass grafting (CABG) and/or an acute coronary syndrome (ACS) within the 3 months prior to study inclusion; a full fasting lipid profile available from within the 12 months prior to study inclusion; and stable use of lipid-lowering therapy (LLT) for at least 3 months prior to enrollment or naivety to LLT. No log was undertaken of patients who were not included based on a lack of a recent lipid profile. By definition, all patients with CHD were considered at very high CV risk according to ESC guidelines [2].
Documentation and definitions
At the index outpatient visit, data regarding patient demographics, clinical characteristics, CHD-related medical history and comorbidities/CV risk factors were documented in a standardized electronic case report form (eCRF). These were physician-based diagnoses, and no further validation of comorbidities was performed. Amongst others, the variables recorded included a sedentary lifestyle (less than 20–30 min of walking on at least 3–4 days per week), hypertension (blood-pressure-lowering treatment, a previous diagnosis of hypertension, or a blood pressure measurement of > 140/90 mm Hg), type 2 diabetes (treatment for diabetes mellitus, a previous diagnosis of diabetes, or a fasting plasma glucose level of ≥ 126 mg/dl), chronic kidney disease (CKD; clinical diagnosis), peripheral artery disease (PAD; clinical diagnosis), and obesity (BMI > 30 kg/m2).
The most recent lipid profile for each patient was also recorded, consisting of total serum cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), and triglycerides. The proportion of patients who had achieved an LDL-C below the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) target of < 1.8 mmol/l for very high-risk CV patients was determined [2]. Although at the time of writing this manuscript the 2019 European guideline has come into effect [3], the 2016 European guideline [2] was chosen for this purpose, as it was the one available around the time of patient recruitment. We felt this to be appropriate as guideline release and general consideration of these recommendations in clinical practice come with a delay of several years.
The type and combination of LLT being prescribed to each patient at the beginning of the study visit, including statin (atorvastatin, rosuvastatin, simvastatin and fluvastatin) and non-statin (ezetimibe and fibrates) agents, was documented. Statin dosages were normalized to atorvastatin potency for the analysis [17]. Any changes to LLT made during the index study visit were recorded.
Statistical analysis
Based on an expected 20–60% of patient LDL-C values being ≥ 1.8 mmol/l, the required sample size needed to evaluate the prevalence of hyperlipidemia with ±1.25% precision (95% CI range: 2.5%) was calculated to be 350 participants. However, the study was designed to provide a descriptive snapshot of real-world clinical practice and all other statistical calculations are for explorative purposes only.
Data are presented using descriptive statistics, with categorical variables expressed as absolute numbers with percentages (%) and continuous variables as means with interquartile ranges or mean with standard deviations. Multivariable logistic regression was performed to identify factors predictive of LDL-C < 1.8 mmol/l target achievement, using the following co-variables: age ≥ 69 years, gender, obesity, large waist circumference, sedentary lifestyle, stable angina, CKD, type 2 diabetes, history of CHF, history of MI, prior PCI or CABG, hypertension, ezetimibe, statin dose, and statin + non-statin combination, statin dose > 20 mg/day atorvastatin equivalent. Results are presented as odds ratios (ORs) with 95% confidence intervals (CI) and p-values. An α level of 0.05 was used to denote statistical significance. TIBCO Spotfire S+ version 8.2 (TIBCO Software Inc., 3307 Hillview Avenue, Palo Alto, CA 94304, USA) was used for all the statistical analyses.
Results
Patient characteristics
A total of 349 patients with stable CHD at very high CV risk were enrolled across outpatient clinics in Slovakia. The mean age was 67.8 years, with the majority of patients being male (67.9%) (Table I). The mean BMI was 29.5 kg/m2, the waist circumference 100.5 cm, and the systolic/diastolic blood pressure 134.5/77.8 mm Hg.
Table I
Baseline demographics and clinical characteristics (n = 349)
Treated or untreated hypertension was the most prevalent CV risk factor (91.7%), followed by a sedentary lifestyle (50.6%), type-2 diabetes (41.0%), and smoking (current 9.5% or history 19.4%). Stable angina was present in 67.7% of patients, with a history of myocardial infarction (MI) in 65.3%, prior PCI in 59.2% and prior CABG in 24.1%.
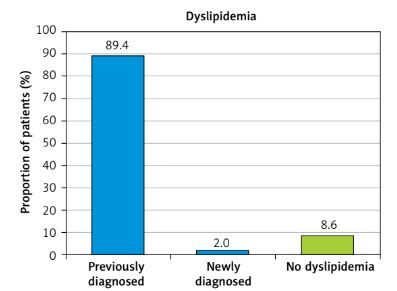
Dyslipidemia
Overall, 89.4% of patients had been diagnosed with dyslipidemia at a prior visit, with a further 2.0% diagnosed at the index study visit (Figure 1). The mean value of all patients was within the normal limits for TC (4.44 mmol/l), and desirable for HDL-C (1.20 mmol/l, and triglycerides (1.68 mmol/l) (Table II). However, the mean LDL-C level was high considering the presence of heart disease: 2.69 mmol/l.
Table II
Lipid profile and concomitant disease markers (mean ± SD and median [IQR])
| Parameter | All CHD patients (n = 349) | LDL-C target* achieved (n = 65) | LDL-C target* not achieved (n = 284) | P-value (target achieved vs. not achieved) |
|---|---|---|---|---|
| Lipid profile [mmol/l]: | ||||
| TC | 4.44 ±1.18 4.24 [3.61, 5.03] | 3.28 ±0.54 3.33 [2.87, 3.68] | 4.70 ±1.12 4.48 [3.88, 5.25] | < 0.0001 |
| LDL-C | 2.69 ±1.02 2.53 [1.97, 3.2] | 1.47 ±0.29 1.57 [1.25, 1.70] | 2.97 ±0.92 2.77 [2.24, 3.46] | < 0.0001 |
| HDL-C | 1.20 ±0.35 1.16 [0.96, 1.40] | 1.22 ±0.44 1.09 [0.93, 1.38] | 1.20 ±0.32 1.17 [0.97, 1.40] | 0.5108 |
| Triglycerides | 1.68 ±0.92 1.50 [1.10, 2.00] | 1.41 ±0.76 1.27 [0.89, 1.66] | 1.74 ±0.95 1.53 [1.15, 2.04] | 0.0014 |
| Other disease markers: | ||||
| Serum glucose [mmol/l] (n = 324) | 6.68 ±2.09 5.90 [5.34, 7.41] | 6.96 ±2.19 5.91 [5.40, 7.99] | 6.62 ±2.07 5.90 [5.31, 7.20] | 0.3524 |
| HbA1c (%) (n = 23) | 6.84 ±1.00 6.9 [6.28, 7.47] | 6.98 ±0.85 7.18 [6.19, 7.58] | 6.76 ±1.09 6.9 [6.45, 7.05] | 0.7463 |
| Serum creatinine [µmol/l] (n = 323) | 94.1 ±67.2 84.0 [73.0, 99.5] | 97.2 ±29.9 88.0 [76.0, 110.3] | 93.4 ±73.6 84.0 [72.2, 96.4] | 0.0291 |
Existing LLT
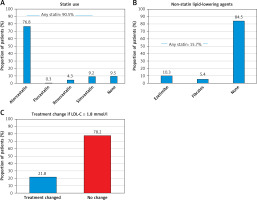
At the start of the index study visit, 90.5% of all patients were being prescribed a statin. The most common statin was atorvastatin (76.8%), with only small proportions taking fluvastatin (0.3%), rosuvastatin (4.3%), and simvastatin (9.2%) (Figure 2 A). The median atorvastatin equivalent statin dose used was 40 mg with an IQR of 20 to 80 mg (mean: 43.8 ±25.4 mg). The majority of patients received no non-statin lipid-lowering agent (84.5%), with 10.3% receiving ezetimibe and 5.4% fibrates (Figure 2 B). The median (only) ezetimibe dose was 10 mg. The median fibrate dose was 165 mg (IQR: 145 to 241 mg) (mean: 196 ±51.7 mg).
Figure 2
Lipid-lowering therapy. A – Type of statin treatment; n = 349. B – Type of non-statin lipid-lowering agents used (n = 349); 1 patient received both ezetimibe and fibrates. C – Lipid-lowering therapy modification during the study visit in patients failing to meet the < 1.8 mmol/l LDL-C target
Target achievement
Only 65 (18.6%) patients achieved an LDL-C < 1.8 mmol/l target. The median LDL-C was 1.57 (IQR: 1.25–1.70) in patients meeting the target vs. 2.77 (IQR: 2.24–3.46) in those failing to achieve the target. Similarly, the median values for TC (3.33 (IQR: 2.87–3.68) vs. 4.48 (IQR: 3.88–5.25) mmol/l; p < 0.0001), and triglycerides (1.27 (IQR: 0.89–1.66) vs. 1.53 (IQR: 1.15–2.04); p = 0.0014) were all significantly lower compared to those who failed to meet the target (Table II).
Upon a multivariable analysis (Table III) of factors potentially associated with improved LDL-C target achievement, age ≥ 69 years (aOR = 3.40; 95% CI: 1.77–6.53) and a statin dose of > 20 mg/day atorvastatin equivalent (aOR = 2.20; 95% CI: 1.13–4.28) were associated with an increased chance of treatment target attainment, while patients with female gender had a reduced chance (aOR = 0.48; 95% CI: 0.24–0.99). Further variables selected for the model based on clinical relevancy had no association with treatment target achievement.
Table III
Univariable and multivariable predictors of LDL < 1.8 mmol/l
| Parameter | LDL-C target* achieved (n = 65) % | LDL-C target* not achieved (n = 284) % | Univariable OR (95% CI) | P-value | Multivariable OR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Age ≥ 69 years | 69.2 | 46.8) | 2.56 (1.44-4.54) | 0.001 | 3.40 (1.77-6.53) | 0.0002 |
| Female gender | 27.7 | 33.1 | 0.77 (0.43–1.41) | 0.401 | 0.48 (0.24–0.99) | 0.046 |
| Obesity** | 35.4 | 42.6 | 0.75 (0.43–1.31) | 0.311 | 0.59 (0.30–1.17) | 0.132 |
| WC > 102 cm (m), > 88 cm (f) | 56.3 | 53.6 | 1.11 (0.65–1.92) | 0.700 | 1.56 (0.76–3.17) | 0.225 |
| Sedentary lifestyle | 56.9 | 49.1 | 1.37 (0.79–2.34) | 0.276 | 1.27 (0.69–2.35) | 0.438 |
| Stable angina | 63.1 | 68.8 | 0.77 (0.44–1.36) | 0.374 | 0.67 (0.36–1.25) | 0.211 |
| Chronic kidney disease | 18.5 | 11.3 | 1.78 (0.86–3.69) | 0.119 | 1.08 (0.45–2.64) | 0.861 |
| Type 2 diabetes mellitus | 44.6 | 40.1 | 1.20 (0.70–2.07) | 0.509 | 0.89 (0.47–1.66) | 0.704 |
| History of congestive heart failure | 32.3 | 18.0 | 2.18 (1.20–3.98) | 0.011 | 2.02 (0.98–4.17) | 0.057 |
| History of MI | 67.7 | 64.8 | 1.14 (0.64–2.02) | 0.657 | 1.02 (0.54–1.93) | 0.960 |
| Prior intervention (PCI or CABG) | 83.1 | 75.6 | 1.58 (0.78–3.19) | 0.202 | 1.35 (0.61–2.99) | 0.459 |
| Hypertension | 92.3 | 91.5 | 1.11 (0.41–3.02) | 0.842 | 1.03 (0.33–3.17) | 0.962 |
| Ezetimibe | 3.1 | 10.9 | 0.26 (0.06–1.11) | 0.069 | 0.47 (0.04–5.43) | 0.543 |
| Statin + non-statin combination | 4.6 | 14.4 | 0.29 (0.09–0.96) | 0.042 | 0.57 (0.08–4.30) | 0.587 |
| Statin dose (> 20 mg/day atorvastatin eq.) | 70.8 | 55.6 | 1.93 (1.08–3.46) | 0.027 | 2.20 (1.13–4.28) | 0.020 |
LLT modification
In patients with an LDL-C ≥ 1.8 mmol/l (n = 284) treatment was adjusted in 21.8% (n = 62) and remained unchanged in 78.2% (n = 222) (Figure 2 C). Of those patients with a treatment change, 32 received atorvastatin, 16 simvastatin and 3 rosuvastatin. Non-statin lipid-lowering treatment was given to 32 patients with more patients receiving ezetimibe (n = 28) versus fibrates (n = 4). Even after the index visit there were patients who were previously uncontrolled and who received no therapy (8.5%) at all (Table IV). Only one patient among those with an LDL-C < 1.8 mmol/l had a treatment change, receiving atorvastatin.
Table IV
Lipid-modifying therapy at study visit
| Parameter | All patients (n = 349) % | LDL-C target* achieved (n = 65) % | LDL-C target* not achieved (n = 284) % | P-value |
|---|---|---|---|---|
| Statin therapy | 91.1 | 100 | 89.1 | 0.0026 |
| Statin monotherapy | 78.5 | 95.4 | 74.6 | < 0.0001 |
| Non-statin therapy | 14.6 | 4.6 | 16.9 | 0.0104 |
| Non-statin monotherapy: | ||||
| Fibrates | 1.1 | 0 | 1.4 | 1.0000 |
| Ezetimibe | 0.3 | 0 | 0.4 | 1.000 |
| Statin + ezetimibe | 9.2 | 3.1 | 10.6 | 0.0597 |
| Statin + other non-statin | 3.4 | 1.5 | 3.9 | 0.7039 |
| Statin in combined therapy | 12.6 | 4.6 | 14.4 | 0.0365 |
| No therapy | 6.9 | 0.0 | 8.5 | 0.0116 |
Discussion
According to the recommendations of ESC, all patients with documented CHD fall under the category of a very high CV risk, and thus the target value in observed patients was < 1.8 mmol/l [2].
Alongside genetic factors, the development of atherosclerotic changes in arteries and subsequent complications such as ACS are closely connected with lifestyle, smoking, suboptimal eating habits, and a lack of exercise, as well as psychosocial stress. A modern view of the prevention of cardiovascular complications consists of an appropriate connection between a population-wide approach and an individual approach focused on high-risk individuals. The population-wide approach consists in particular of government-coordinated activities aimed at the modification of (among other things) lifestyle, reduction of the intake of fats and salt from food, smoking ban campaigns, and legislative measures. Such activities have potentially the highest impact on the reduction of cardiovascular complications amongst the population. The individual approach is aimed at high-risk individuals, especially those in the care of healthcare professionals.
This study was the first to describe the profile of Slovak patients regularly treated for the chronic form of CHD. It must be stressed that according to the protocol, the diagnosis of CHD had to be very clear and substantiated by a history of ACS, documented coronary revascularization, or coronary angiography. Modification of risk factors contributes by at least 50% to the reduction of CV mortality when speaking in terms of the whole society, and analyses from European countries performed using the IMPACT model stress in particular the importance of reducing cholesterol levels [5, 6, 12–14]. However, the reduction of CV mortality in Slovakia in the previous 20 years does not reach the level of neighboring countries (Poland, Hungary, Czech Republic, Austria), and the analysis of the reduction of mortality in Slovakia according to the IMPACT model revealed only a 43% share of the benefit resulting from the modification of risk factors [7]. While the acute management and hospital mortality of patients with ACS in Slovakia nowadays reach global parameters, medium- and long-term mortality is very unfavorable. These findings are very suggestive for the issues regarding secondary prevention and they may explain one of the main reasons for the long-term unfavorable position of Slovakia in the European statistics of so-called avoidable deaths. The management of hyperlipoproteinemia was one of the main topics of the DYSIS II study, and the findings point to the fact that society and healthcare professionals owe much to patients with a very high CV risk. According to the evidence-based medicine and official professional recommendations by ESC, the target LDL cholesterol in these patients is ≤ 1.8 mmol/l. The fact that only 18.6% of patients reach the target LDL value is alarming, but comparable with data from abroad. In the international DYSIS II study, which mapped 30 countries in the category of patients with a very high CV risk, 29.4% of patients reached the target LDL value ≤ 1.8 mmol/l [4, 15]. This was based on an average atorvastatin dose of 25 ±18 mg/day [4]. Failure to reach therapeutic goals in Slovakia is even more surprising when we take into account the fact that almost 3/4 of patients taking atorvastatin were on high doses (40 mg or 80 mg/day). The fact that despite having learnt that a patient does not reach the target LDL value in many patients the outpatient care specialist did not switch to a different treatment in terms of dosing or dose structure casts a bad light. The current ESC recommendations in the case of failure to reach target LDL levels despite the maximum tolerable dose of statins is to add ezetimibe [2, 18, 19]. In the subgroup of patients reaching the target LDL value, there were more elderly patients (aOR = 3.40) and more men (aOR = 1.52), possibly implying that these conditions increased the attention paid by physicians to the more consistent control of lipid levels.
The principal purpose of the current study was to establish a nationally representative picture on the real-world lipid levels in Slovakia outpatients, and we approached 35 sites nationwide which each recruited 10 patients. We acknowledge, however, that the patient numbers actually recruited can only be a proper approximation of the current situation with ±1.25% precision. A limitation of this study is that the analysis is based on lipid values found in the medical records and not the analysis of blood samples and that adherence to treatment was not directly assessed. Although most patients reported receiving a statin prescription at follow-up, actual medication-taking behavior was not queried. Secondly, the follow-up was too short to observe any changes in therapy or cardiovascular outcomes. A long-term follow-up of the patients would be of interest to evaluate any changes of therapy in the longer term of the disease. Finally, data collection by telephone at follow-up may not have been as accurate as the medical chart review used for data collection at admission and may have affected the direction and degree of changes observed over time.
There is a clear need for improving lipid-target attainment in patients with dyslipidemia. This can be achieved by promoting clinical guidance such as presented by the European Society of Cardiology in 2016 [2] and further refined in the most recent 2019 guideline [3]. Uptake of clinical guideline may be, however, poor and severely delayed, and new iterations are usually associated with even stricter and more refined treatment targets. Hence there is potential for clinical practice to be disconnected from experts writing the guidelines. To overcome this, a group of specialists from Poland, for example, recently summarized the treatment of hypercholesterolemia also to non-specialists, as patients are usually first seen by this physician group and appropriate action can be taken early [20]. Furthermore, clear guidance was developed from this specialist group for the laboratory diagnostics of lipid metabolism disorders to improve standardization of laboratory values used to treat dyslipidemia [21].
Furthermore, efforts to improve lipid control must not be confined to LDL-C target level achievement, but need to take into account the role of hypertriglyceridemia [22]. It further requires careful exploration of patient subgroups potentially also having a benefit from lipid-lowering treatment such as patients with atrial fibrillation and cardioembolic stroke [23]. Oral statins may even improve diseases not commonly associated with statin use such as psoriasis, particularly in patients with severe disease [24]. Research has also shown that metformin may stabilize PCSK9 levels in patients with CAD treated with statins [25].
In conclusion, patients at high cardiovascular risk in Slovakia are far off the < 1.8 mmol/l LDL-C level recommended for protection from adverse events, and a lower percentage achieve their treatment targets (18.6%) than the mean of other countries globally (29.4%). While in-hospital care and outcomes may be reasonably in line with other European countries, the persistent long-term management of patients appears to be key in reducing hyperlipoproteinemia-associated cardiovascular events in Slovakia.