Introduction
The coronavirus disease 2019 (COVID-19) pandemic has become a significant crisis affecting all countries worldwide [1]. COVID-19 is caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which belongs to the B lineage of β-coronaviruses and is closely related to the SARS-CoV virus. SARS-CoV-2 is 96% identical to a bat coronavirus at the whole-genome level [2, 3]. Whole-genome sequencing and phylogenic analysis indicate that SARS-CoV-2 belongs to the betacoronaviruses located in the Sarbecovirus subgenus along with related species responsible for severe acute respiratory syndrome (SARS), while the Middle East respiratory syndrome (MERS) belongs to the betacoronaviruses located in the subgenus Merbecovirus [4]. These diseases are mainly transmitted through respiratory droplets, with more than half of the patients experiencing cough and shortness of breath, but less than 20% of patients have apparent signs and symptoms of upper respiratory tract infection, such as nasal mucus, sneezing (15%) or sore throat (14%). This indicates that the target of the coronavirus is the lower respiratory tract [5–8].
In China, about 93.6% of COVID-19 patients with the above respiratory symptoms or fever choose to be hospitalized; the median length of hospital stay (interquartile range) is 12 (10–14) days. About 15.7% of these patients have a severe course of the disease, and their average mortality rate is as high as 8.1% [9]. Evidence for an increasing number of cases of COVID-19-related neuropsychiatric symptoms is emerging [10]. The incidence of neuropsychiatric symptoms, such as insomnia, anxiety, depression, and mania, is also increasing in influenza patients. SARS-CoV and MERS-CoV, which are homologous to SARS-CoV-2, have been reported to cause several neuropsychiatric sequelae, including seizures, Guillain-Barre syndrome, and narcolepsy [11–15]. Talarowska et al. consider mood disorders and depressive episodes to be related to the gene expression of the peripheral inflammatory response and oxidative balance indicators. They also believe that the onset of depression is a kind of immune inflammation, and oxidative stress disorder is related to progressive changes in the nervous system [8]. It seems that when patients are faced with such a high risk of severe illness and mortality, invisible pressure will make them increasingly worried about their situation. Excessive anxiety and tension in patients with COVID-19 and other diseases usually herald poor disease progression and treatment prognosis [16]. Therefore, paying attention to the mental health of patients during their treatment can be beneficial.
With the increasing emphasis on socio-psycho-biomedical models, prognosis and quality of life have become essential evaluation indicators in clinical work. COVID-19 patients in isolation wards commonly exhibit signs of anxiety and depression and display symptoms such as irritability, despair, abnormally low mood, and discomfort [17]. The existing literature has investigated the mental health problems caused by the stress response of ordinary healthy people and medical workers to the epidemic [18–20]. It has been found that women are more psychologically affected than healthy uninfected people, and on average, are moderately anxious. Research has also shown that young people aged 18 to 30 and older adults over 60 are more vulnerable to psychological effects [21], and people with a high level of education tend to be more anxious [19]. Although it is understood that an individual’s personality will make a difference to the stress response [22], it is not known whether, when the patient is diagnosed with stress, the emotional abnormality caused by the stress will affect the development of the disease. There needs to be further research into what factors affect emotional changes.
The Hospital Anxiety and Depression Scale (HADS) was devised in 1983 by Zigmond and Snaith [23]. It has been translated into numerous languages. Some studies have shown it to be the most effective and reliable tool for diagnosing anxiety and depression in individuals with disease [24, 25]. It consists of questions about feeling concerned with everyday life and aims to quantify symptoms including nervousness, worry, fear and panic, difficulty relaxing, and unease. The Insomnia Severity Index (ISI) was developed by Weaver et al. in 1997 [26]. Effective indicators and studies have confirmed that the ISI is a reliable and sensitive indicator for detecting insomnia in clinical patients with disease [27].
This study was carried out in Hangzhou, China. Its purpose is to help clinicians identify the emotional changes in COVID-19 patients. Based on the research results, the aim is to clarify whether a patient has emotional abnormalities when the index is higher or lower, so the appropriate psychological counseling and guidance, or even drug intervention, can be provided. It is essential to be particularly vigilant concerning the deterioration of the COVID-19 condition. Thus, when patients appear to show a sudden mood disorder or personality traits contrary to the usual, clinicians should remember to treat the symptoms and provide mental health advice. The difficulties associated with clinical treatment are an important factor affecting the prognosis of the disease. Therefore, psychological interventions have come to play a vital role in the treatment of the disease. However, at present, there are no complete data on the degree and prevalence of anxiety and depression among patients with COVID-19. In particular, there have been no reports on the relationship between the degree of anxiety and depression, the severity of COVID-19, and patients’ blood tests.
Material and methods
General features
This research met the ethical requirements and was approved on March 11th, 2020, by the ethics committee of Xixi Hospital of Hangzhou in Zhejiang province, China (No. LKYJ-2020-08). From January 23rd, 2020, to February 29th, 2020, data on adult (aged 18 years or over) COVID-19 patients admitted to Xixi Hospital of Hangzhou – the authorized hospital for treating COVID-19 in Zhejiang province – were collected.
Diagnosis criteria
At the time of the study, the diagnosis met the Guidelines for the Diagnosis and Treatment of Novel Coronavirus Infection, and the criteria were as follows: (1) contact with infected areas or confirmed patients 14 days before onset; (2) fever or upper respiratory symptoms; (3) lung computed tomography (CT) showing multiple small patchy shadows and interstitial changes, multiple ground-glass shadows or infiltrations in both lungs, lung consolidation, or pleural effusion in some severe cases; and, (4) the total number of white blood cells in the early blood test normal or decreased, and reduced lymphocytes. The suspected cases met any two of the above-mentioned criteria.
The confirmed cases were based on the determination of the suspected cases and met either of the following criteria: (1) respiratory tract specimens or blood specimens tested positive for the new coronavirus nucleic acid with real-time fluorescent RT-PCR; (2) the viral gene sequencing was highly homologous with the known coronavirus [28].
COVID-19 was clinically classified into three types: mild, moderate, and serious. The diagnostic criteria of those three types were as follows: mild: mild clinical symptoms, no pneumonia on imaging; moderate: fever, respiratory symptoms, pneumonia on imaging; serious: respiratory distress, a blood oxygen saturation at rest of ≤ 93%, and an arterial blood oxygen partial pressure/inhaled oxygen concentration of ≤ 300 mm Hg.
Inclusion criteria
We included patients who could communicate in-depth in their native Chinese language and answer the questions posed in the questionnaire, had been in contact with a confirmed case and became a suspected patient, and had received a final diagnosis of COVID-19 after participating in centralized isolation.
Exclusion criteria
This research’s exclusion criteria were patients with psychotic disorders, severe cognitive impairment, and central or peripheral nervous system disease. In addition, patients with a history of epilepsy (except for pediatric convulsions), deaf-mute patients or patients with a language disability, and patients with multiple organ dysfunction syndromes accompanied by severe cardiac insufficiency, severe liver and kidney damage, or other serious organ damage, were excluded.
Questionnaires and grouping
After a definitive diagnosis of COVID-19, the patients completed HADS and ISI scales and questionnaires, which were administered via self-report or by an interviewer. The researcher explained the instructions for filling in the forms but avoided asking any suggestive or tendentious questions to ensure the accuracy and objectivity of the results. Moreover, it was monitored so that the patients chose an answer corresponding to the right question. Due to the particularity of the isolation ward, the investigators were the attending physicians of those patients. Before starting the study, these physicians underwent training, which included familiarization with all the questions on the questionnaire, and learned how to provide “unanswered explanations” when the patients did not understand the questions they had read. The investigators also read the questions and options out loud to 9.6% of the patients who had dyslexia and, thus, a low level of literacy. The patients then made their choice by themselves, and the remaining 90.4% filled out the questionnaire on their own. The questionnaires were completed on the 3rd–5th days and the questionnaire assessment was conducted within 3 days of a definitive diagnosis of COVID-19, each of the measures typically requiring 5 min to complete. Basic information about the patients was collated, including their gender, age, education level, smoking status, lymphocyte count, C-reactive protein (CRP), leukocyte count, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and chest computed tomography (CT).
The patients were divided into three groups according to age: under 40, 40–55, and over 55 years of age.
Evaluation criteria
Scoring was accomplished by adding up the scores for different items on the questionnaires, with particular attention paid to reversed items. The total score range for the HADS-A was 0–21. The following guidelines were recommended to interpret scores: 0–7 for normal or no anxiety; 8–10 for mild anxiety; 11–14 for moderate anxiety; and 15–21 for severe anxiety. The same method was used for the HADS-D [24]. ISI was scored according to a 7-point Likert scale, and the items were added together to determine the total score. The score range was 0–28 points, with higher scores indicating greater insomnia severity. The suggested guideline for score interpretation was as follows: 0–7 for no clinically significant insomnia; 8–14 for subthreshold insomnia; 15–21 for clinical insomnia (moderate severity); and 22–28 for clinical insomnia [29].
Statistical analysis
For the statistical processing, SPSS 24.0 was used to calculate the basic information and scale results. T-tests (two-category) for HADS scores were performed on baseline data belonging to categorical variables to compare the differences in anxiety scores and depression scores between different genders, educational backgrounds, smoking habits, and those with underlying diseases. The differences in anxiety scores and depression scores among different ISI groups were compared. Nonparametric rank-sum tests did not satisfy normality, and the nonparametric rank-sum test was used to compare the differences in anxiety and depression among different COVID-19 grades. P < 0.05 was considered statistically significant. The analysis of variance (ANOVA) was used to compare anxiety scores and depression scores among different age groups. Differences in depression scores, differences in leukocytes and lymphocytes between different lung infections, and differences in anxiety, depression, and ISI scores between different lung infections, and the anxiety and depression levels between different COVID-19 grades were all compared using the nonparametric rank test. The Pearson correlation coefficient is a statistic used to reflect the degree of linear correlation between two variables. The Spearman rank correlation was used to describe the correlation between the indicators. Either Spearman or Pearson correlation analysis was used to study the correlation between CRP, lymphocytes, white blood cells, ALT, AST, anxiety and depression scores, and the correlation between anxiety scores and depression scores.
Results
General features
The study consisted of 52 COVID-19 patients (aged 23–64, average age: 40.8 ±11.8), more than half (57.7%) of whom were female. After being screened using the HADS, 59.6% were diagnosed with anxiety and 36.5% with depression. Basic information about the patients is shown in Tables I and II.
Table I
Demographics and baseline characteristics of the patients (n = 52)
Table II
Blood morphology and biochemical results of the patients (n = 52)
Comparative analysis of anxiety/depression scores with disease severity, sleep patterns, and certain laboratory test results
The HADS-A scores of patients who smoked or had basic diseases were higher than those who did not. There was no significant difference in HADS-A scores between different genders or those with varying levels of academic qualifications (Table III). Similarly, depression is more likely to exist in patients with an underlying disease. The anxiety scores of patients aged 40–55 years and over were higher than patients under 40. HADS-D scores in patients aged 40–55 years and over were higher than in those under 40 (Table IV). In addition, the HADS-D scores of patients with the basic disease were higher than in those without the disease. There was no significant difference in HADS-D scores between different genders, educational backgrounds, or smoking status. No direct relationship was found between education levels and HADS-A or HADS-D scores. COVID-19 caused fear and anxiety in patients of all educational levels, irrespective of their understanding of the disease.
Table III
Comparison of anxiety scores between different basic data (n = 52)
| Basic data | HADS-A scores | t/F | P-value | |
|---|---|---|---|---|
| Gender | Male | 8.32 ±4.03 | 0.016 | 0.987 |
| Female | 8.30 ±4.01 | |||
| Age | Under 40 | 6.48 ±3.34 | 6.287 | 0.004 |
| 40-under55 | 10.29 ±4.33* | |||
| 55 and above | 9.50 ±2.88* | |||
| Education background | High school and below | 8.47 ±4.50 | 0.334 | 0.740 |
| Bachelor and above | 8.09 ±3.22 | |||
| Smoking | Yes | 11.33 ±3.54 | 2.654 | 0.011 |
| No | 7.67 ±3.80 | |||
| Previous disease | Yes | 10.90 ±2.47 | 2.397 | 0.020 |
| No | 7.69 ±4.04 |
Table IV
Comparison of depression scores between different basic data (n = 52)
| Basic data | HADS-D scores | t/F | P-value | |
|---|---|---|---|---|
| Gender | Male | 5.41 ±3.53 | 0.583 | 0.562 |
| Female | 6.00 ±3.67 | |||
| Age | Under 40 | 4.40 ±2.80 | 3.817 | 0.029 |
| 40-under55 | 6.88 ±4.33* | |||
| 55 and above | 7.20 ±2.97* | |||
| Education background | High school and below | 5.87 ±3.79 | 0.271 | 0.787 |
| Bachelor and above | 5.59 ±3.38 | |||
| Smoking | Yes | 7.67 ±4.06 | 1.802 | 0.078 |
| No | 5.35 ±3.39 | |||
| Previous disease | Yes | 8.40 ±2.55 | 2.765 | 0.008 |
| No | 5.12 ±3.53 |
The CRP level in bilateral infection was higher than it was in unilateral infection or no infection. The invasion of CRP in the lungs of COVID-19 is more sensitive, and the CRP level of infections in both lungs is significantly higher than that of unilateral infections and groups without lung infection images. CRP, lymphocytes, leukocytes, ALT, AST, and anxiety did not statistically significantly correlate with anxiety and depression scores. There were no statistically significant differences in leukocytes and lymphocytes between different lung infections. There were no statistically significant differences in anxiety, depression, and ISI scores among those with different lung infections (Table V). There was also no significant difference in the level of anxiety between different subtypes. It shows that the degree of lung infection in imaging is not significantly related to the patient’s mood changes or sleep disturbance. In clinical practice, we found that even mild or healthy patients may be excessively anxious about their condition due to the environmental impact.
Table V
Comparison of leukocytes, CRP, lymphocytes and anxiety, depression, and ISI scores among different lung infections (n = 52)
| Chest CT | CRP | Lymphocytes | Leukocytes | HADS-A scores | HADS-D scores | ISI scores |
|---|---|---|---|---|---|---|
| No infection | 1 (1,9) | 1.49 ±0.73 | 7.03 ±2.73 | 8.14 ±3.67 | 5.43 ±3.60 | 5.00 ±4.36 |
| Unilateral infection | 1 (1,12) | 1.57 ±0.83 | 5.90 ±2.01 | 6.71 ±3.04 | 4.41 ±2.87 | 4.82 ±4.93 |
| Bilateral infection | 10 (4.25,25.25)* | 1.16 ±0.43 | 6.27 ±2.65 | 9.32 ±4.33 | 6.64 ±3.81 | 7.79 ±4.46 |
| F/x2 | 9.986 | 2.540 | 0.529 | |||
| F | 2.421 | 2.176 | 2.585 | |||
| P-value | 0.007 | 0.089 | 0.593 | 0.099 | 0.124 | 0.086 |
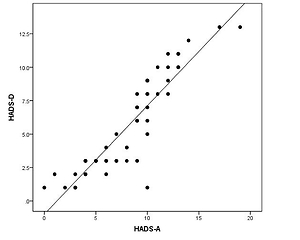
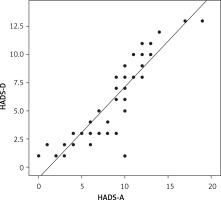
The correlation between HADS-A and HADS-D scores was statistically significant. The correlation coefficient was 0.908, indicating a positive correlation between the HADS-A score and the HADS-D score (Figure 1). This means that patients with depression often have accompanying anxiety, and depressive tendencies often accompany patients with higher anxiety scores.
The difference in HADS-A and HADS-D scores between the different ISI groups was statistically significant. Groups with ISI scores less than eight had lower HADS-A and HADS-D scores than groups with ISI scores of eight or more (Table VI). It shows that the better the sleep is, the lower is the probability of emotional disturbance.
Table VI
Comparison of HADS-A and HADS-D between different ISI groups (n = 52)
| ISI group | HADS-A | HADS-D |
|---|---|---|
| Under 8 | 4.95 ±2.46 | 2.91 ±1.66 |
| 8 and above | 10.77 ±2.94 | 7.83 ±3.16 |
| t | 7.528 | 6.650 |
| P-value | < 0.001 | < 0.001 |
According to the results, clinicians should pay special attention to patients’ emotional state when encountering COVID-19 patients over 40 years old with underlying diseases and/or poor sleep quality. When a patient’s anxiety score increases, pay special attention to whether he/she is depressed. This study shows that patients often have some symptoms of anxiety before the onset of depression.
Discussion
The results showed that the number of patients selected before mid-February was far more than that after mid-February. The first reason was that the transmission frequency of SARS-Cov-2 was markedly reduced by the latter part of February due to government control measures. The second reason was that the media and the internet widely publicized COVID-19 to make people understand the disease and take precautions more consciously. At the same time, it was also found that the anxiety and depression of patients diagnosed at the early stage of the disease outbreak were significantly higher but decreased after that, which may be related to the guidance of the official media. In the first 2 weeks of the outbreak, 16.5% of the general population had moderate to severe depressive symptoms, and 28.8% had moderate to severe anxiety symptoms [19].
Inflammatory factors are independent factors in patients with generalized anxiety [30]. Tayefi et al. divided 9,759 anxiety patients into four groups of no or minimal, low, moderate and severe categories and analyzed their high-sensitivity C-reactive protein (hs-CRP) levels. They found that the serum hs-CRP concentration correlated positively with the severity of anxiety and depression, and women with severe anxiety and depression had higher body mass index values [31]. Although there are many reports that elevated CRP is significantly associated with anxiety [32], the results of this study show that the correlation between CRP and anxiety and depression is not statistically significant. This may be due to the small sample size and large deviation. Although there is no evidence that lymphocytes are directly related to anxiety and depression, lymphocytes can reflect the severity of COVID-19. Tayefi et al. also concluded that patients with severe COVID-19 have moderate anxiety, indirectly reflecting the psychological relationship between emotional abnormalities and disease severity [31]. Ko et al. found that AST in patients with liver cirrhosis is closely related to anxiety, depression, or insomnia [33], but this study did not confirm that. The possible reasons are as follows. (1) Although multiple organs may be invaded by COVID-19, the leading cause of anxiety, etc., is respiratory and lung infections. (2) There are many causes of acute liver injury, with viral infections and drug abuse being the most common, but whether the transient increase in transaminases is related to neuropsychiatry is still unknown. (3) AST is an important mediator in the inflammatory process of hepatitis and cirrhosis, and increasing evidence has shown that the pathophysiological process of depression is closely related to pro-inflammatory factors [34].
The emotional disorders caused by COVID-19 occur not only in patients who have been diagnosed with the disease but also in healthy people who are isolating. Many countries and governments have advocated home isolation to reduce the spread of SARS-CoV-2 despite a large number of reports that isolation can cause psychological distress such as upset, anxiety, insomnia, exhaustion, and even depression [35, 36], predominantly impacting children and their parents. Parents bear the responsibility for their families’ health and economy and worry about social distancing between their children and their children’s peers and teachers. In addition, some parents cannot fulfill their children’s educational needs [37]. An Italian survey showed that only 6.1% of children’s families have at least one computer [38]. It may be good that parents can spend more time with their children during the epidemic, but this may also bring new pressures, and parents’ lack of patience, for example, may lead to fragile relationships [39]. Members of the general public lack an in-depth understanding of COVID-19 and may be exposed to exaggerated reports and false information in the media, leading to excessive pessimism among those diagnosed with or suspected of having COVID-19. For those reasons, experts have also studied whether people in areas where COVID-19 is not endemic will be more susceptible to a kind of unrealistic optimism. The results show that men, in particular, are generally optimistic that they will not be infected, suggesting that such people could spread COVID-19 by violating the precautionary measures of wearing a mask and keeping a distance recommended by medical experts [40].
Studies have demonstrated that medical staff also face great mental stress when treating COVID-19 patients. Sources of stress include the high risk of infection, inadequate contamination protection measures, overwork, frustration, discrimination, isolation, patients’ negative emotional states, not being able to see their families, and physical loss. These factors can cause a range of mental health problems among health care professionals, such as stress, anxiety, depression, insomnia, denial, anger, and fear. These problems affect the attention, understanding, and decision-making ability of medical staff, which, in turn, affects the prevention and control of the pandemic and has lasting adverse effects on their overall health. Therefore, protecting the medical staff’s mental health is essential to controlling the disease’s spread [41].
The government has taken various measures to combat the mental health problems outlined above. For example, medical staff infected by SARS-CoV-2 at work are identified as having a work-related injury. According to statistics published by the National Health and Medical Commission, by February 12th, 2020, the commission had sent a total of 189 medical teams, including 21,569 medical staff, to support medical treatment in Hubei Province [42]. These medical teams provided medical care to confirmed and suspected patients, boosted logistical protection, and reduced front-line pressure. In addition, various online platforms provided suggestions about reducing cross-infection in patients or medical institutions and how to prevent and control COVID-19 in patients, the ultimate goal being to reduce pressure on the medical staff. The government has also introduced medical insurance for patients diagnosed with the disease to relieve patients’ financial stress. However, at present, no professional psychological team interventions are available; instead, it is the role of clinical front-line medical staff to help ease patients’ emotional stress. However, the question is whether, when they are under psychological pressure themselves, they are able to give their patients the help they need concerning their emotional and mental well-being.
This study has several limitations. Firstly, this experiment is a single-center study. In addition to disease, other factors may also impact patients’ emotional state, including the hospital environment and their relationship with their doctors. Future studies should include case studies not only from Zhejiang or Hubei provinces but the rest of the country, too, to provide a more comprehensive view of the emotional status of COVID-19 patients during their illness. Secondly, in this research, questionnaires were completed on the 3rd–5th day by the selected patients, but there was no comparison with the anxiety and depression scales of in-hospital patients and those leaving the hospital. Therefore, this study is correlative and by itself cannot conclude on causation. It is unclear whether patients’ depression and anxiety during hospitalization were related to stress alone or to the disease itself. Further research should investigate the relationship between anxiety and depression and COVID-19, using additional data from a follow-up scale survey after patients have been discharged.
In conclusion, this study aims to help clinicians understand more comprehensively the anxiety and depression manifestations of COVID-19 patients. The results of the present study indicate that patients of a specific age who sleep poorly, smoke, and have an underlying illness are more likely to suffer from anxiety and depression. At the same time, anxiety and depression tend to occur together and show a positive correlation. In addition, the inflammatory index CRP was significantly increased in bilateral lung infections. With COVID-19 patients over 40 years of age who are seen in the clinic and have accompanying underlying diseases, smoking, or lack of sleep, the staff must be vigilant, perform corresponding emotional screenings when necessary, and provide emotional counseling in time.