Introduction
Hearing loss, also known as auditory impairment or hearing impairment, abbreviated as HL, is a type of auditory disorder characterized by the reduction or degradation of hearing, leading to partial or complete loss of auditory function [1]. According to statistics, in the year 2013, approximately one billion people worldwide suffered from varying degrees of HL [2]. The number of individuals with disabling HL was estimated to be around 5%, with approximately 124 million experiencing moderate to severe disability [3]. In 2019, the World Health Organization (WHO) calculated that about 1.57 billion individuals worldwide were afflicted by HL, and projections show that this number is expected to exceed 2.45 billion by 2050 [4]. Extensive research on the global burden of disease has revealed that hearing loss ranks as the third most prevalent cause of disability on a global scale [5]. Assessing the economic implications linked to the years affected by hearing loss-induced disability, recent data suggest that these costs could potentially exceed USD 750 billion annually [6]. Consequently, with the expanding absolute number and proportion of the aging demographic, hearing loss is set to emerge as a significant contributor to disability.
HL may occur in one or both ears and can be of temporary or permanent nature. In children, HL may impact language acquisition, while in adults, it can result in challenges in the workplace [7]. For some individuals, HL may lead to a sense of isolation, especially among the elderly population [8]. Potential causes of HL include genetics, aging, exposure to noisy environments, infections, complications related to childbirth, ear trauma, and certain medications or toxins [9]. Some previous observational clinical investigations have suggested that body constitution-related phenotypes are abnormal in HL patients [10], highlighting the significance of body constitution-related phenotypes in the progression of HL.
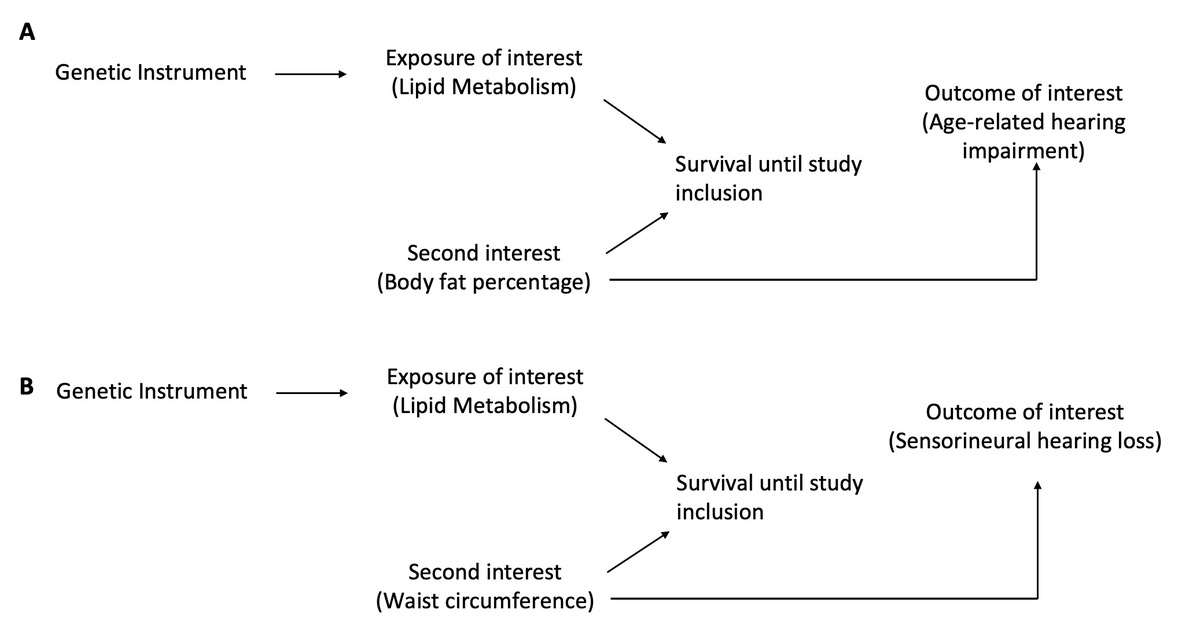
Considering the considerable societal and economic challenges linked to hearing loss, there is an increasing inclination to identify changeable elements, such as dietary habits, that may have an impact on auditory function and perhaps reduce the likelihood of hearing impairment [11, 12]. Over the past decade, significant attention has been directed towards fatty acids as a specific dietary component, given their multifaceted implications for human health [13]. Fatty acids play integral roles in cellular membranes, impacting membrane fluidity and, consequently, the function of membrane-bound proteins, including ion channels and receptors [14, 15]. Because of their antioxidant and anti-inflammatory properties, these fatty acids hold promise in potentially preventing aging-related or noise-induced hearing loss. However, studies on the relationship between subtypes of HL and body constitution-related phenotypes are inconclusive and the results are usually conflicting. Hence, there are currently insufficient data to link body constitution-related phenotypes to HL. Prior investigations were of an observational nature, leaving unresolved concerns related to residual confounding, survival bias, selection bias and reverse causation. In contrast, the Mendelian randomization (MR) approach stands out for its enhanced resilience against unmeasured confounding and reverse causation, issues that often compromise precise causal inferences in traditional epidemiological studies. In response to these concerns, we conducted a genetic MR investigation with the goal of determining the possible causative link as well as the direction in which bodily composition and hearing loss are related. Considering the likelihood of both backward (from hearing loss to body constitution) and forward (from body constitution to hearing loss) causation, we formulated a hypothesis positing a bi-directional causal connection.
Material and methods
We performed a bidirectional two-sample Mendelian randomization (MR) analysis. From publicly accessible summary data of IEU OPEN genome-wide association studies (GWAS), genetic relationships for every trait were derived (https://gwas.mrcieu.ac.uk/) (Table I). To mitigate potential biases stemming from weak instruments because of sample overlap [15], whenever possible, we looked for GWAS datasets with non-overlapping samples. Given the robust signal within the SLC39A8 gene region for age-related hearing impairment (ARHI) genetic connections and body mass index (BMI), colocalization analysis was done before the MR analysis. This was done in an effort to examine whether the correlation is due to confounding by linkage disequilibrium (LD) or whether the signal originates from a common causal variation.
Table I
Sources of secondary summary statistics employed in the derivation of instrumental variables for the assessment of adult body constitution and its association with HL
Data sources for body constitution-related phenotypes and hearing loss-related phenotypes
Measurements of the adult body constitution included body fat percentage (BFP) (%), waist circumference (WC) (cm), and BMI (kg/m2). For BMI and WC, the data were obtained from the Genetic Investigation of Anthropometric Traits (GIANT) encompassing 322,154 and 245,746 individuals respectively reported in 2015. The data of BFP were derived from UK Biobank, with a sample size of 454,633 participants, published in 2018.
Hearing loss traits included ARHI, sensorineural hearing loss (SNHL) and sudden idiopathic hearing loss (SIHL). The GWAS on risk of ARHI (sample size = 330,759) was from the UK Biobank, reported in 2020. Summary-level genetic data for risk of SNHL (15,952 cases and 196,592 controls) and SIHL (1,491 cases and 196,592 controls) were obtained from the FinnGen project including 212,544 and 198,083 participants, respectively.
Statistical analysis
In the inverse variance weighted (IVW), MR-Egger, weighted median, and weighted mode MR analyses, the number of single nucleotide polymorphisms (SNPs) serves as the count for genetic instrumental factors, along with the F-statistic for each exposure-outcome pair. When considering SNHL and SIHL as exposure variables, a p-value threshold of 5.00E-05 was set due to the limited data available (compared to the threshold of 5.00E-08 for other variables).
Colocalization analysis
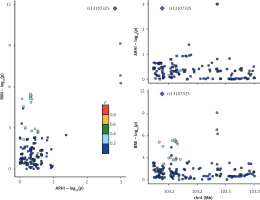
Variants at the ferroptosis-related gene SLC39A8 locus (chr4:103172237 -103352415, hg19) [16] were linked to both ARHI and BMI, although it was unclear whether the causative variation for both traits was the same. The colocalization analysis was performed using the ‘coloc’ method formulated by Giambartolomei et al. [17], supposing that each genomic site can only have one causal variant. Colocalization was assumed to be present in model H4 if the PP was more than 0.7 [18], as shown in Table II.
Results
Colocalization analysis
Figure 1 and Table II display the findings of the colocalization investigation between BMI and ARHI. At the SLC39A8 locus, we discovered a common causal variation (rs13107325) for ARHI and BMI, which is supported by a PP for H4 of 0.99. The A allele of the variant rs13107325 was found to be positively correlated with BMI and negatively correlated with ARHI. When this variant alone was included in the ARHI BMI estimation, the MR estimate was 0.75 (95% CI: 0.63 to 0.89). The allelic variant at the rs13107325 locus exhibits a positive association with BMI (p = 1.83E-12, p < 5.00E-08; R = 0.98) and an association with ARHI (p = 0.0014, R = 0.90).
Forward MR analyses
The findings of the analysis are shown in Figure 2 (left panel) and Supplementary Table SI. Our findings indicated a lack of evidence supporting the idea that none of the HL-related analysis results were linked to genetically predicted BMI. For the risk of ARHI, SNHL, and noise-induced hearing loss (NIHL), the corresponding IVW ORs for increase in exposure were 1.00 (95% CI: 0.97 to 1.03), 1.10 (95% CI: 0.95 to 1.27), and 1.09 (95% CI: 0.73 to 1.62) respectively. Likewise, we could find no evidence that HL outcomes were related to genetically determined WC. For NIHL and ARHI, the corresponding IVW ORs were 1.20 (95% CI: 0.77 to 1.85) and 0.99 (95% CI: 0.84 to 1.20), respectively. Furthermore, there was no discernible correlation between BFP and SNHL (OR = 0.95 (95% CI: 0.84 to 1.08), OR = 1.19 (95% CI: 0.86 to 1.64)) or NIHL. Lastly, we found that BFP was positively correlated with the risk of ARHI (OR = 0.95 (95% CI: 0.93 to 0.98)), and that WC was correlated with the risk of SNHL (OR = 0.83 (95% CI: 0.72 to 0.97)).
Figure 2
The forest plots display causal estimates, represented as OR, along with their corresponding 95% CI. The left panel shows forward analyses (from body constitution-related outcomes to subtypes of HL). Conversely, the right panel exhibits the reverse analysis (from subtypes of HL to body constitution-related phenotypes)

The sensitivity analysis estimates matched the IVW when analyzing the correlation between WC liability and SNHL, as illustrated in Figure 3 and Supplementary Table SII. There was not much indication of directional pleiotropy shown by the MR-Egger intercept (intercept = 0.00, p = 0.49). Contrarily, the outcomes from the weighted median and weighted mode methods diverged from the IVW results in the analysis of the association between WC liability and SNHL.
Figure 3
Scatter plots that illustrate the estimates from Mendelian randomization sensitivity analyses in the context of forward two-sample Mendelian randomization

The estimates derived from IVW-aligned sensitivity analyses in examining the relationship between BFP liability and ARHI are illustrated in Figure 3 and Supplementary Table SIII. There was little indication of directed pleiotropy as shown by the MR-Egger intercept (intercept = 0.00, p < 0.001). Contrarily, the outcomes from the weighted median and weighted mode methods diverged from the IVW results in the analysis of the association between WC liability and SNHL.
The individual SNP funnel plot indicates balanced pleiotropy by showing a comparatively symmetrical distribution of SNP effects around the impact estimates (Supplementary Figure S1). Additionally, a leave-one-out analysis was performed (Supplementary Figure S2), yielding no evidence of a single SNP exerting disproportionate influence on the results.
Reverse MR
The analysis’ findings are shown in Figure 2 and Supplementary Table SII. Our findings demonstrated that the hypothesis linking ARHI to any of the body composition outcomes examined in these analyses was unsupported by the available data. The final IVW impact sizes for BMI, WC, and BFP were 1.57 (95% CI: 0.76 to 3.24), 0.86 (95% CI: 0.68 to 1.08) and 1.04 (95% CI: 0.87 to 1.25), respectively. Furthermore, the observed impact sizes for BMI, WC, and BFP were 1.02 (95% CI: 1.00 to 1.04), 1.01 (95% CI: 0.99 to 1.03), and 1.00 (95% CI: 0.99 to 1.01), respectively. No evidence was discovered linking predicted SNHL to outcomes of body constitution. Lastly, our results, which showed the observed effect sizes of 1.00 (95% CI: 0.99 to 1.01), 0.99 (95% CI: 0.99 to 1.00), and 1.00 (95% CI: 1.00 to 1.00), respectively, did not support the hypothesis that SIHL was related to BMI, WC, and BFP. Additionally, the sensitivity analyses (Figure 4 and Supplementary Table SIV) did not agree with the IVW.
Discussion
This research used a wide range of complementary sensitivity analyses and two-sample Mendelian randomization procedures to investigate the causal relationship between body constitution features and HL. The findings suggest that there may be a causal risk-increasing impact behind the often-seen positive correlation between body composition and hearing loss in observational research. Our investigation revealed causal associations between WC and SNHL, as well as between BFP and ARHI in prospective Mendelian randomization analyses. Notably, the SLC39A8 variant emerged as a common factor contributing to both high BMI and the risk of ARHI.
SLC39A8 variants are potentially linked to an increased risk of ARHI
The colocalization analysis indicated a common causal variant, rs13107325, within the SLC39A8 locus for both BMI and ARHI. The gene SLC39A8, situated on chromosome 4q24, is responsible for encoding the manganese (Mn) transporter ZIP8. ZIP8 is pivotal in the maintenance of manganese homeostasis within both blood and tissues [19]. In a GWAS involving 123,900 individuals, BMI was evaluated. Subsequently, 42 single nucleotide variants (SNVs) were specifically investigated in approximately 125,900 additional individuals. This analysis revealed associations between BMI and 14 previously identified obesity-susceptibility loci, as well as 18 novel loci. Notably, one of these loci implicated the SLC39A8 p. Ala391Thr variant (p < 1.5 × 10–13) in relation to increased BMI [20]. Given the extensively documented association between SLC39A8 variants and BMI, a plausible scenario emerges where BMI may exert influence on ARHI. Our findings suggest that individuals carrying the SLC39A8 gene variant rs13107325 may be susceptible to ARHI. Our findings first indicated a new role of the SLC39A8 gene variant; however, the mechanism driving this association remains elusive and requires further investigation [21]. SLC39A8 serves as a shared gene implicated in both obesity and brain function, with previous research robustly validating the link between obesity variants and brain volumes [22]. Notably, the SLC39A8 gene is associated with the sensorimotor system, providing partial corroboration for the outcomes of this study. A joint GWAS analysis, followed by enrichment for traits related to hearing and body constitution, represents a complementary approach that could offer deeper insights into shared biological pathways. Subsequent studies are imperative to unravel the intricate mechanisms from the perspective of comorbidity/multimorbidity.
Analysis of the evidence regarding a potential causal relationship wherein body constitution influences outcomes of HL
Using the body of knowledge already available from observational studies and biological plausibility, our hypothesis posits that heightened BFP, WC and BMI may contribute to the onset of hearing disorders. In the IVW analysis, we identified a positive association between WC and SNHL, as well as between BFP and ARHI. Furthermore, sensitivity analyses indicated that these associations were not influenced by horizontal pleiotropy. Collectively, our findings provide some support for these causal relationships with both ARHI and SNHL, recommending that alterations in body constitution, possibly induced by fluid mentalism, could elucidate prior observations. An earlier study also suggested that excessive adiposity might impact hair cells in the inner ear’s cochlea [23]. Consequently, applying proxies to the distribution of fat, such as BFP and WC, may enhance the conclusion that some forms of hearing loss are caused.
Analysis of the evidence regarding a potential causal relationship wherein HL influences outcomes of body constitution
Within this investigation, reverse MR analyses yielded scant evidence supporting a causal relationship between HL and outcomes related to body constitution. The GWAS boasted substantial sample sizes, and the narrow confidence intervals of effect sizes suggested ample statistical power for detecting associations. It is crucial to highlight that, due to the rarity of NIHL and SNHL in the FinnGen data, we adjusted the p-value threshold to enhance the availability of instrumental variables. Still, NIHL prevalence is considerably greater among the broader populace, with estimates indicating around 12.5% among children [24] and 17% among adults aged 20 to 69 years [25]. Consequently, the outcomes from FinnGen might not be fully appropriate for the larger population.
WC and BFP may increase the risk of SNHL and ARHI respectively
Our findings imply genetic proof indicating that a person’s inclination to higher WC and BFP could be linked to SNHL and ARHI were confirmed by the sensitivity studies of MR. It aligns with findings from a recent, sizable study carried out in a cohort from the population of West Azerbaijan reporting a positive association between metabolic syndrome and SNHL [26]. Previous results showed that participants with SNHL had a higher number of components of metabolic syndrome (p < 0.001 for all components) [26]. Another early study, involving 690 adults with normal or symmetrical SNHL, aimed to explore the effect of central adiposity on the intensity and characteristics of ARHI [23]. The results suggest that WC, independently of BMI, serves as a distinct risk factor for ARHI, especially for low and high frequencies in males under 55 and for high frequencies in females over 55 [23]. The previous results are similar to our current findings, indicating that a high WC and BFP due to body constitution-related phenotypes can increase some HL phenotypes.
Prevention and measures
There are several ways to reverse adverse health outcomes, such as daily activities, exercise, or approved medications. Regular exercise is essential for maintaining a healthy body weight and reducing the risk of adverse health outcomes [27]. Engaging in aerobic exercises such as brisk walking, jogging, swimming, or cycling helps burn calories and improve cardiovascular health. Strength training exercises also promote muscle growth and metabolism. Additionally, adopting a balanced and nutritious diet plays a crucial role in achieving and maintaining normal body size [28]. There should be a focus on consuming whole grains, fruits, vegetables, lean proteins, and healthy fats while limiting processed foods, sugary beverages, and high-calorie snacks. Moreover, for individuals with obesity-related health issues, approved medications prescribed by healthcare professionals can complement lifestyle modifications [29]. These medications may help reduce appetite, enhance satiety, or prevent fat absorption, depending on individual health needs.
Limitations of the study. Several limitations merit discussion. Hearing loss is sensitive primarily to significant alterations in receptor quality, and substantial changes in constitution may yield inadequate power for positive MR owing to genetic variations’ generally modest impact sizes. While MR offers greater robustness compared to traditional epidemiological methods regarding unmeasured confounders, it is crucial to acknowledge the potential bias introduced by unobserved environmental confounders [30]. Future research endeavors could be designed to explore the hearing implications for underweight, overweight, or obese individuals, considering the potential for nonlinear effects in establishing causality.
In conclusion, there was no evidence that we could uncover to support a causal relationship between constitution and HL in the reverse MR analysis. Colocalization analysis revealed that high BMI and ARHI risk were often caused by the SLC39A8 mutation. We discovered causal relationships in prospective MR between WC and BFP with SNHL and ARHI, respectively. Therefore, our results suggest that lipid metabolic abnormalities leading to adverse health outcomes may lead to HL.