Introduction
Laryngeal squamous cell carcinoma (LSCC) is the one of the most common cancers within men worldwide, increasing by about 600,000 cases per year [1], which is caused by drinking and smoking and other possible risk factors such as human papilloma virus (HPV) and Epstein-Barr virus (EBV) infections, work environment and nutrition [1, 2]. A survey shows that there are about 9,500–11,000 new laryngeal cancer patients in the United States [3]. At present, larynx-conserving surgery and radiotherapy are effective strategies aiming at the therapy of the cancer, and combined therapy acts on advanced laryngeal cancer, including adjuvant therapy [4, 5]. However, the mortality rate of LSCC remains high [6]. Under this circumstance, some researchers have studied the biomarkers of LSCC. Schluter et al. reported that CD31 and vascular endothelial growth factor (VEGF) are prognostic biomarkers [7]. Other studies indicated that miR-93-5p [8] and miR-196b [9] are potential markers in LSCC. However, studies on long non-coding RNAs (lncRNA) are rare in LSCC, while lncRNA is a critical regulator of coding RNA and alternative splicing, and it is associated with tumorigenesis, tumor progression, and metastasis [10]. Therefore, it is particularly urgent to search for new biomarkers of LSCC to carry out early diagnosis.
Long non-coding RNAs are RNA molecules that are between 200 and 100,000 nucleotides (nt) long and which are incapable of being translated into proteins [11, 12]. Research has shown that lncRNAs regulated crucial cancer pathways at epigenetic, transcriptional, and post-transcriptional levels [13]. Meanwhile, reports showed that abnormal lncRNA expression has been found in tumors. The lncRNA HEIH showed higher transcript levels in hepatitis B virus-related hepatocellular carcinoma (HCC). The lncRNA JADE has markedly higher levels in human breast tumors. Simultaneously, aberrant transcript profiles of lncRNAs were observed in non-neoplastic diseases, such as ventricular septal defect and acute renal rejection [14]. Numerous studies have indicated that lncRNA was involved in the regulation of various processes in the cell, such as cell growth, proliferation, differentiation, survival and apoptosis [15, 16]. Therefore, scientists started to discover that imbalance of lncRNAs leads to the evolution of tumor through adjusting these cell processes [13, 17]. Besides, lncRNA expression had not only cellularity and tissue specificity, but also some lncRNA were expressed only in specific stages of eukaryotic development [18].
B-Raf proto-oncogene (BRAF)-activated non-coding RNA (BANCR) has been confirmed to have a close relationship with some human cancers [19]. Research indicated that BANCR has abnormal expression in some cancers, such as colorectal cancer [20], melanoma [21], gastric cancer [22], lung carcinoma [23], and hepatocellular carcinoma [24]. For example, Shi et al. found that BANCR inhibited colorectal cancer cell growth via interaction with p21 protein, and it demonstrated that BANCR was a regulator of the pathogenesis in colorectal cancer [20]. Also, BANCR promoted cell proliferation in lung carcinoma and malignant melanoma by regulating the mitogen-activated protein kinase (MAPK) pathway [23, 25]. Above all, BANCR was a potential biomarker of early diagnosis, prognosis, and potential therapy targets in laryngeal carcinoma [26]. However, the expression and function of lncRNA BANCR in LSCC are unclear.
Cisplatin has been widely used in many solid tumors, is one of the most effective and common drugs for the treatment of a wide spectrum of tumors, and patients usually get effective results in the early stages of using cisplatin [27]. However, the overuse of therapy accelerated the process of generating drug tolerance, leading to an obstacle for a favorable therapeutic outcome [28]. After pathogens, such as HPV and EBV, make contact with the drug many times, the sensitivity of tumor cells was reduced or even disappeared, resulting in the reduction or invalidation of the drug to the pathogens [2, 29]. Drug resistance of tumor cells is the central barrier for the medical domain to treat laryngeal carcinoma, and continuous use of the drug often hinders treatment results [16, 30].
In this study, we altered BANCR expression by transfection to explore the role of BANCR in the progression of laryngeal carcinoma cell. The results revealed that downregulation of BANCR expression enhanced cell sensitivity to cisplatin via inhibiting cell proliferation of LSCC.
Material and methods
Tissue samples and cell culture
In total, 47 specimens of human LSCC tissues and adjacent tissues were obtained from the Tianjin Union Medicine Centre (Tianjin, China). Samples were allocated into two groups: an experimental group (tumor tissues) and a control group (adjacent tissues). The study was performed according to the institutional ethical guidelines. The ethics committee of Tianjin Union Medicine Centre approved a written informed consent form which was signed by every patient, and all the consents were saved by the ethics committee. The conduct of the study was in accordance with the principles expressed in the Declaration of Helsinki. All samples were snap-frozen in liquid nitrogen, then stored at –80°C for further use.
Normal esophageal cell Het-1A and laryngeal squamous carcinoma cells TU686 and TU177 came from BeNa Culture Collection (Beijing, China). Cells were cultivated in Roswell Park Memorial Institute (RPMI) 1640 culture medium supplemented with 10% fetal bovine serum (FBS, TBD, Tianjin, China), 100 units/ml penicillin G and 100 µg/ml streptomycin and incubated in a 5% CO2 humidified atmosphere at 37°C. Cisplatin (DDP)-resistance (R) laryngeal squamous carcinoma cells (TU686-DDP-R, TU177-DDP-R) came from TU686 and TU177 cells with continuous cisplatin treatment.
Western blotting
Cells were harvested and lysed in radioimmu-noprecipitation assay buffer. Protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride (PVDF) membranes. After blocking with 5% skimmed milk, the membranes were incubated overnight at 4°C with primary antibodies, followed by incubation with secondary antibodies.
Quantitative reverse transcription-polymerase chain reaction (QRT-PCR) assay
Total RNA was isolated from tissues and cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). All steps were performed under RNase-free condition. A UV spectrophotometer (Bio-Rad, California, USA) was used to detect RNA concentration and optical density (OD) value. The reverse transcription reactions were carried out via a PrimeScript RT reagent kit (RR047A, TaKaRa Bio Inc., Japan). For quantitative real-time PCR (qPCR), a final volume of 20 µl reaction was performed according to a standard protocol and the SYBR Green PCR kit (Roche Diagnostics, Indianapolis, IN, USA) on the StepOnePlus RealTime PCR System (Applied Biosystems, Carlsbad, CA, USA). The qPCR was performed in triplicate, with no template controls. The 2–ΔΔCT method was conducted to determine the relative gene expression levels and the endogenous control was glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The QRT-PCR was performed using the following cycles: 95°C for 30 s; 40 cycles of 95°C for 5 s and 60°C for 31 s; and a dissociation stage at 95°C for 15 s, 60°C for 1 min and 95°C for 15 s. The following were the primer (Sangon biotech, Shanghai) sequences: BANCR primers: 5’-ACAGGACTCCATGGCAAACG-3’ (forward) and 5’-ATGAAGAAAGCCTGGTGCAGT-3’ (reverse). GAPDH primers: 5’-GCACCGTCAAGGCTGAGAAC-3’ (forward) and 5’-TGGTGAAGACGCCAGTGGA-3’ (reverse).
SiRNA and plasmid transfection
The specific small interfering RNA that targeted BANCR (siRNA-BANCR) and a scrambled negative control (siRNA-NC) came from GenePharma (Shanghai, China). The expression vector (pcDNA3.1) expressing BANCR (pcDNA3.1-BANCR) and a scrambled negative control (pcDNA3.1) were also constructed and purchased from Gene-Pharma (Shanghai, China). The cells were cultured 24 h prior to transfection. Then, the cells were transiently transfected with corresponding siRNA or pcDNA3.1 by using Lipofectamine 2000 Transfection Reagent (Invitrogen, Carlsbad, CA, USA) on the basis of the manufacturer’s instructions. Cells transfected with siRNA or pcDNA3.1-BANCR were harvested for further analysis after 48 h. Small interfering RNAs can guide Argonaute-containing RNA-induced silencing complexes to target RNAs in a sequence-specific way, leading to mRNA deadenylation followed by exonucleolytic decay, mRNA endonucleolytic cleavage, or translational inhibition [31]. pcDNA 3.1 is 5.4 kb vector and is designed for high-level expression. For high-level expression, it contains the human cytomegalovirus (CMV) immediate-early promoter. High-level expression can be performed in most mammalian cells [32].
Cell counting
The cells were cultured 24 h prior to transfection. Cells were treated with expressing BANCR (pcDNA3.1-BANCR), a scrambled negative control (pcDNA3.1) and cultured for 48 h, then cells were collected through trypsinization, mixed in PBS and trypan blue was added. Cells were counted using a microscope counting chamber.
CCK-8 assay
A reagent of Cell Counting Kit-8 (CCK-8) assay (Beyotime, Institute of Biotechnology, Shanghai, China) was used in the cell proliferation assay. 3000 viable cells per well a 96-well tissue culture plates in a final volume of 100 µl were used. Every 24 h, a plate was subjected to assay by adding 10 µl of CCK-8 solution to each well, and the plate was further incubated for 4 h at 37°C. The Microplate Photometer was used to measure the absorbance at 450 nm. All assays were performed in triplicate and all experiments were repeated three times.
Colony formation assay
The method of the colony formation assay: a total of 1000 cells were plated into each well of a 6-well plate and cultured in medium containing 10% FBS incubation at 37°C in 5% CO2 for 2 weeks, replacing the medium every 4 days. The colonies were fixed with methanol, stained with Giemsa, and counted. Triplicate wells were measured in each treatment group.
Statistical analysis
Data were analyzed using GraphPad Prism 6 and were expressed as the mean ±SD. The significance of the differences between groups was estimated by Student’s t-test, or one-way analysis of variance, as appropriate. Two-sided p < 0.05 was deemed to show a statistically significant difference.
Results
LncRNA BANCR expression level was high in tumor tissues and LSCC cells
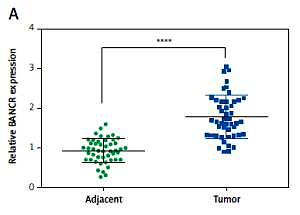
The relative BANCR expression level was detected in tumor tissues and adjacent normal tissues from 47 patients via using QRT-PCR. It was notable that BANCR expression was higher in tumor tissues compared with adjacent normal tissues (Figure 1 A). Compared with the Het-A cells, the BANCR expression was increased significantly in LSCC cells TU686 and TU177 (Figure 1 B). To evaluate LSCC cell growth after overexpression BANCR, we estimated cell counts. As shown in Figure 1 C, the cell count was significantly higher in the pcDNA3.1-BANCR group, compared with the pcDNA3.1 group. In order to confirm the prognostic value of BANCR expression for laryngeal squamous cell carcinoma, we acquired the Kaplan-Meier survival curves for the correlation between the levels of BANCR expression and overall survival from The Cancer Genome Atlas (TCGA), and we found that BANCR expression was significantly correlated with laryngeal squamous cell carcinoma patients’ overall survival (Figure 1 D). Patients with increased BANCR expression had worse overall survival than those with low expression of BANCR. The above data demonstrated that lncRNA BANCR may function as an actuator gene in LSCC. Thus, it can be seen that BANCR had a certain relationship with LSCC.
Figure 1
Expression of BANCR in tumor tissues and laryngeal squamous cell carcinoma (LSCC) cells. A – The expression of BANCR was examined by qRT-PCR, and 47 samples of tumor tissues and adjacent tissues were investigated. ****P < 0.0001, compared with adjacent tissues. B – Expression of BANCR in LSCC cells and laryngeal cells was determined by qRT-PCR. **P < 0.01, compared with Het-1A. C – Cell counting after pcDNA3.1-BANCR transfection in TU686 and TU177 cell lines. Cell count was markedly increased in pcDNA3.1-BANCR group, compared with pcDNA3.1 control. D – The association between overall survival rates and BANCR expression
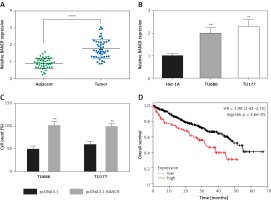
The level of BANCR was overexpressed in cisplatin-resistant LSCC cells
Initially, we established cisplatin-resistant cell lines TU686-DDP-R and TU177-DDP-R. TU686 and TU686-DDP-R cells, and TU177 and TU177-DDP-R cells were treated with various concentrations of DDP (1, 2, 4, 8, 16, 32 µg/ml) for 48 h. Then, the cell viability was examined (Figures 2 A, B). The results showed that cell viability of TU686 was significantly lower compared with TU686-DDP-R cells with DDP treatment. Similar results appeared in TU177 cells and TU177-DDP-R cells. Colony formation assays indicated that the colony numbers of TU686-DDP-R and TU177-DDP-R cells with 10 µg/ml DDP treatment were obviously higher than TU686 and TU177 (Figures 2 C, D). As shown in Figure 2 E, we observed that the expression of BANCR in TU686-DDP-R and TU177-DDP-R was significant higher compared with TU686 and TU177. It indicated that high BANCR expression may make cells become resistant to cisplatin in LSCC.
Figure 2
The cisplatin-resistant cell line was established. A, B – TU686 and TU686-DDP-R cells, and TU177 and TU177-DDP-R cells were treated with various concentrations of DDP (0, 1, 2, 4, 8, 16, 32 μg/ml). C, D – Colonyforming growth assays were performed to determine the proliferation of TU686, TU177, TU686-DDP-R, TU177-DDP-R cells with 10 μg/ml DDP treatment. The colonies were counted and captured. E – The expression of BANCR in TU686, TU686-DDP-R, TU177, TU177-DDP-R was observed by qRT-PCR assay. Data represent the mean ± SD. **P < 0.01, compared with TU686 or TU177
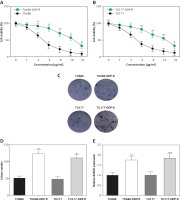
Downregulation of BANCR increased sensitivity of cisplatin-resistant LSCC cells by inhibiting proliferation in vitro
BANCR expression levels were enhanced by transfecting pcDNA-BANCR in TU686 and TU177 cells, while the expression of BANCR was inhibited via transfecting with siRNA-BANCR in TU686- DDP-R and TU177-DDP-R. QRT-PCR analysis of BANCR expression levels suggested that BANCR expression was enhanced in TU686 and TU177 cells following transfection with pcDNA-BANCR compared with pcDNA3.1 (Figure 3 A). Relative expression levels of BANCR in TU686-DDP-R and TU177-DDP-R with siRNA-BANCR were fewer than si-NC (Figure 3 B). Moreover, cell viability of LSCC cells with pcDNA3.1-BANCR transfection was significantly high following treatment with various concentrations of DDP (1, 2, 4, 8, 16, 32 µg/ml) compared with the pcDNA3.1 group (Figures 3 C, D), while cell viability of DDP-resistant LSCC cells with siRNA-BANCR was clearly lower compared with si-NC (Figures 3 E, F). Representative data of experiments showed that upregulated BANCR reduced sensitivity of TU686 and TU177 cell to cisplatin. In contrast, the cisplatin-resistant cell line became sensitive after downregulation of BANCR expression.
Figure 3
The effect of BANCR expression on sensitive cells and cisplatin-resistant cells in laryngeal squamous cell carcinoma (LSCC). A – Relative expression of BANCR in TU686 and TU177 after transfection of pcDNA3.1 and pcDNA3.1-BANCR. **P < 0.01, compared with pcDNA3.1. B – Relative BANCR expression of TU686-DDP-R, TU177-DDP-R after transfection of siRNA-BANCR and si-NC. **P < 0.01, compared with si-NC. C, D – CCK-8 assay was used to evaluate sensitivity of TU686 and TU177 to DDP after transfection of pcDNA3.1-BANCR. All cells were administered with different concentrations of cisplatin for 24 h. **P < 0.01, compared with pcDNA3.1. E, F – CCK-8 assay was used to evaluate sensitivity of TU686-DDP-R and TU177- DDP-R to DDP after transfection siRNA-BANCR. **P < 0.01, compared with si-NC
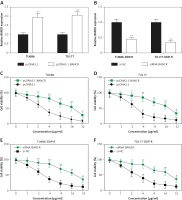
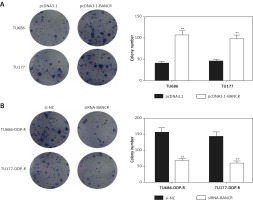
Downregulation of BANCR increased sensitivity of cisplatin-resistant LSCC cells by inhibiting colony formation in vitro
The proliferative impact of BANCR on the process of cells was further assessed by colony formation assays. Colony numbers of the pcDNA3.1-BANCR group were obviously higher than the pcDNA3.1 in TU686 and TU177 cells with 5 µg/ml DDP treatments. However, colony numbers of the siRNA-BANCR group were lower than si-NC in TU686-DDP-R and TU177-DDP-R with 5 µg/ml DDP treatment (Figures 4 A, B). The observations indicated that downregulation of BANCR increased sensitivity of cisplatin-resistant LSCC cells.
Figure 4
Sensitivity of cells to DDP was examined by colony-forming growth assays. A – The level of clone formation in TU686 and TU177 with pcDNA3.1-BANCR or pcDNA3.1 transfection and DDP treatment in vitro. The colonies were counted and captured. **P < 0.01, compared with pcDNA3.1. B – After treatment with siRNA-BANCR and DDP, the colonies of TU686-DDP-R and TU177-DDP-R were counted. **P < 0.01, compared with si-NC. All cells were treated with 10 μg/ml DDP
Modulation of MRP1 and apoptosis-related genes
To investigate through which BANCR cell viability/formation is regulated, we detected the expression of MRP1, Bcl-2, p-PKB and Bax. Bcl-2 is anti-apoptotic protein, while Bax plays an important role in proapoptotic processes. They exist in intrinsic apoptotic pathways [33]. Further, protein kinase B (PKB) is an important member of the PI3/PKB pathway. The activated PI3K/PKB pathway was found in head and neck carcinomas and phosphorylated protein kinase B (p-PKB) was increased [34]. Moreover, multidrug resistance is a major contributor to the failure of chemotherapy in patients suffering from multidrug resistance-associated protein 1 (MRP1) was up-regulated in laryngeal cancer [35, 36]. Our results showed that the expression of MRP1, Bcl-2, p-PKB of LSCC cells with pcDNA3.1-BANCR transfection was significantly high compared with the pcDNA3.1 group (Figures 5 A–C, E–G), while the expression of Bax was decreased in the pcDNA3.1-BANCR group (Figures 5 D, H). In addition, MRP1, Bcl-2, and p-PKB expression was reduced in DDP-resistant LSCC cells with siRNA-BANCR compared with si-NC (Figures 5 I–K, L–N). Conversely, the Bax expression was increased (Figures 5 E, O). These results indicated that the PI3/PKB signaling pathway may be related to cell viability, which was regulated by BANCR. Bcl-2 and Bax may participate in this process.
Figure 5
The effect of BANCR on the expression of MRP1, Bcl-2, p-PKB, Bax in sensitive cells and cisplatin-resistant cells of laryngeal squamous cell carcinoma (LSCC). The expression of MRP1, Bcl-2, p-PKB, Bax in TU686 (A–D) and TU177 (E–H) with pcDNA3.1-BANCR or pcDNA3.1 transfection. **P < 0.01, compared with pcDNA3.1. MRP1, Bcl-2, p-PKB and Bax expression in TU686-DDP-R (I–L) and TU177-DDP-R (M–P) after transfection of siRNA-BANCR or si-NC. **P < 0.01, compared with si-NC

Discussion
Laryngeal squamous carcinoma is the most common cancer of head and neck squamous cell carcinoma around the world [6, 37], in which lncRNAs occupy an important position. Feng et al. reported that lncRNAs are potential biomarkers in advanced LSCC [38]. Wu et al. showed that LncRNA H19 could accelerate LSCC pathological processes through miR-148a-3p and DNM [39]. Li et al. indicated that lncRNA HOTAIR plays a regulatory role in LSCC [40]. At present, relevant research has found that lncRNA may have a vital effect on different aspects of cell pathology and physiology, such as cancer [41]. Cisplatin (DDP) has a wide anticancer spectrum, strong function, and it has no cross resistance or other characteristics, such as toxicity, and cisplatin can establish a mouse model of acute kidney injury [42]. Further, it is one of the most commonly used drugs in combination chemotherapy at present. However, drug resistance is a serious obstacle to efficient chemotherapy and represents a major problem in clinical oncology [27]. Consequently, gene therapy becomes an appealing and effective option for cancer treatment. The experiments of Zhu et al. demonstrated that the suppression of the cluster of differentiation 147 (CD147) gene as a potential target for therapeutic anti-cancer drugs enhanced chemosensitivity to cisplatin in laryngeal carcinoma human epithelial-2 (Hep2) cells [6]. Also, low LncRNA HOTAIR expression promoted enhancer of zeste homolog 2 (EZH2) expression, contributing to the increase of the sensitivity to DDP in the LSCC cells through promoting the proliferation of AMC-HN8 cells [43]. Xu et al. deemed that apigenin may enhance the sensitivity to cisplatin of laryngeal carcinoma cells via suppressing GLUT-1 and p-Akt genes [44]. Furthermore, some studies indicated lncRNAs, especially BANCR, as potential oncogenes or inhibitor genes to tumors, had crucial regulatory functions in tumorigenesis and tumor progression. Zhang et al. reported that BANCR as a tumor suppressor inhibits aggressiveness in papillary thyroid cancer [45]. Moreover, Wang et al. indicated that BANCR promotes endometrial cancer cell proliferation, migration and invasion [46]. In addition, BANCR had an inseparable relationship with melanomas, papillary thyroid carcinomas, colorectal carcinomas, lung cancers and serous ovarian carcinomas [47, 48], which means that BANCR may serve as a novel biomarker for cancer diagnosis and disease prognosis evaluation. BANCR-targeting inventions may be explored as a promising therapeutic option in cancer treatments [49]. That is, regulating BANCR was a possible orientation to treat tumor, and lncRNA BANCR can be considered as therapeutic targets, at least for some cancer types [50]. However, it was completely unclear for the biological functions of lncRNA BANCR in LSCC.
Research revealed that high BANCR expression emerged in LSCC cells. Similarly, BANCR was overexpressed in malignant melanoma tissues [21]. Conversely, low expression of BANCR had been observed in both lung cancers and colorectal cancers [23]. Also, the research of He et al. revealed that BANCR expression was low in bladder cancer tissues and cells [51]. We have developed a cisplatin-resistant LSCC cell line from human laryngeal carcinoma cells TU686 and TU177 with cisplatin treatment, showing that BANCR was overexpressed in cisplatin-resistant cell lines TU686-DDP-R and TU177-DDP-R based on BANCR overexpressed in LSCC cells. Further, we regulated expression of BANCR to explore whether BANCR expression had an impact on sensitivity of LSCC cells and cisplatin-resistant LSCC cells by influencing cell proliferation after the transfection of pcDNA3.1-BANCR or siRNA-BANCR. Previous research showed that downregulation of BANCR promoted the proceeding of proliferation via inhibiting p21 protein expression in colorectal cancer cells and bladder cancer cells [20, 51]. On the other hand, BANCR accelerated cell proliferation via activating the MAPK pathway in lung carcinoma [25, 52]. Furthermore, down-regulation of BANCR remarkably repressed endometrial cancer cell proliferation via inhibiting the ERK/MAPK signaling pathway that regulates MMP1 and MMP2 [45]. In our research, apparently, on the one hand, elevated BANCR expression protected LSCC cells from the toxicity of cisplatin and promoted cell proliferation in comparison with the control cells. On the other hand, the results showed that knockdown of BANCR repressed proliferation of LSCC cells. Meanwhile, overexpression BANCR increased the expression of p-PKB in LSCC cells, while knockdown of BANCR decreased protein expression levels of p-PKB in resistant cells. Taken together, the results showed that downregulation of BANCR enhanced sensitivity of cisplatin-resistant LSCC cells by inhibiting proliferation in vitro. The PI3/PKB signaling pathway may participate in this process.
In conclusion, BANCR was confirmed to participate in the progression of cells such as proliferation in laryngeal squamous carcinoma. Knockdown of BANCR restrained cell proliferation. Our study demonstrated that sensitivity of cisplatin-resistant laryngeal squamous carcinoma cells could be rescued successfully by downregulating the expression of lncRNA BANCR. The study of specific BANCR biological function has important significance for the diagnosis of cancer and prediction of new target genes, and our studies provide an important theoretical basis for further research.