Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
NEUROLOGY / CLINICAL RESEARCH
Early treatment with ofatumumab increases the likelihood of stabilizing disease in patients with relapsing-remitting multiple sclerosis
1
Military Institute of Medicine - National Research Institute, Warsaw, Poland
2
Saint Luke’s Specialist Hospital, Konskie, Poland
3
Department of Neurology – Clinical Hospital of Karol Marcinkowski’s Medical University, Poznan, Poland
4
Clinic of Neurology, Medical University of Silesia, Katowice, Poland
5
Department of Neurology – Ludwik Rydygier’s Specialist Hospital, Golden Autumn Estate, Krakow, Poland
6
Department of Economic and Medical Informatics, University of Lodz, Lodz, Poland
7
Clinic of Adult Neurology, Medical University of Gdansk, Poland
Submission date: 2024-12-21
Final revision date: 2025-02-04
Acceptance date: 2025-05-14
Online publication date: 2025-06-24
Corresponding author
Aleksandra Pogoda-Wesołowska
Military Institute of Medicine – National Research Institute Szaserów St. 128 04-141, Warsaw, Poland
Military Institute of Medicine – National Research Institute Szaserów St. 128 04-141, Warsaw, Poland
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Methods of multiple sclerosis (MS) treatment are evolving rapidly, with numerous classes of disease-modifying therapies (DMTs). A more aggressive approach to early and effective treatment of MS with a defined treatment target increases the chance of achieving a state of no evidence of disease activity (NEDA). Currently, B cell-depleting monoclonal antibodies have been proven as a highly effective strategy for the treatment of relapsing-remitting MS (RRMS). Ofatumumab (OFA), an anti-CD-20 monoclonal antibody, is effective in treatment of RRMS, as it positively affects relapse rates, magnetic resonance imaging (MRI) measures of disease activity, and disability progression.
Material and methods:
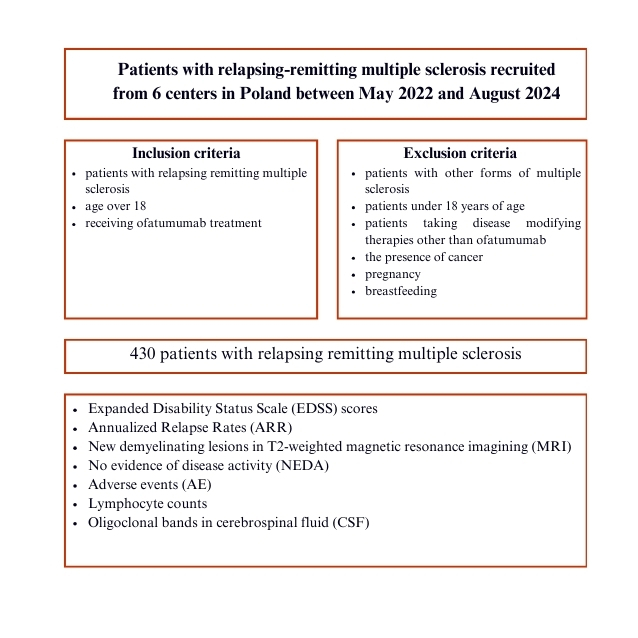
A retrospective observational study was conducted in six MS clinical centers in Poland, including a cohort of patients with RRMS treated with OFA over a 2-year period.
Results:
The results of this study showed a statistically significant decrease in the relapse activity of the disease in the course of a year of OFA therapy. The percentage of patients free of relapses increased from 45% before treatment to 88% after 1 year of follow-up. Moreover, the disability assessment index measured by the Expanded Disability Status Scale (EDSS) remained stable after 2 years of follow-up.
Conclusions:
In the present study, the high efficacy of OFA therapy in reducing recurrent disease activity, as well as in inhibiting disability progression, with a favorable safety profile, was confirmed. Moreover, it was emphasized that to achieve the best possible inhibition of disease activity and its progression, it is necessary to implement the treatment as soon as possible after the diagnosis.
Methods of multiple sclerosis (MS) treatment are evolving rapidly, with numerous classes of disease-modifying therapies (DMTs). A more aggressive approach to early and effective treatment of MS with a defined treatment target increases the chance of achieving a state of no evidence of disease activity (NEDA). Currently, B cell-depleting monoclonal antibodies have been proven as a highly effective strategy for the treatment of relapsing-remitting MS (RRMS). Ofatumumab (OFA), an anti-CD-20 monoclonal antibody, is effective in treatment of RRMS, as it positively affects relapse rates, magnetic resonance imaging (MRI) measures of disease activity, and disability progression.
Material and methods:
A retrospective observational study was conducted in six MS clinical centers in Poland, including a cohort of patients with RRMS treated with OFA over a 2-year period.
Results:
The results of this study showed a statistically significant decrease in the relapse activity of the disease in the course of a year of OFA therapy. The percentage of patients free of relapses increased from 45% before treatment to 88% after 1 year of follow-up. Moreover, the disability assessment index measured by the Expanded Disability Status Scale (EDSS) remained stable after 2 years of follow-up.
Conclusions:
In the present study, the high efficacy of OFA therapy in reducing recurrent disease activity, as well as in inhibiting disability progression, with a favorable safety profile, was confirmed. Moreover, it was emphasized that to achieve the best possible inhibition of disease activity and its progression, it is necessary to implement the treatment as soon as possible after the diagnosis.
REFERENCES (21)
1.
Scolding N, Barnes D, Cader S, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol 2015; 15: 273-9.
2.
Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing remitting multiple sclerosis. N Engl J Med 2008; 358: 676-88.
3.
Gärtner J, Hauser SL, Bar-Or A, et al. Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: results from ASCLEPIOS I and II. Multiple Sclerosis J 2022; 28: 1562-75.
4.
Bar-Or A, Wiendl H, Montalban X, et al. Rapid and sustained B-cell depletion with subcutaneous ofatumumab in relapsing multiple sclerosis: APLIOS, a randomized phase-2 study. Mult Scler 2022; 28: 910-24.
5.
Kira JI, Nakahara J, Sazonov DV, et al. Effect of ofatumumab versus placebo in relapsing multiple sclerosis patients from Japan and Russia: phase 2 APOLITOS study. Mult Scler 2022; 28: 1229-38.
6.
Bar-Or A, Grove RA, Austin DJ, et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: the MIRROR study. Neurology 2018; 90: e1805-14.
7.
Hauser SL, Bar-Or A, Cohen JA, et al. ASCLEPIOS I and ASCLEPIOS II Trial Groups. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med 2020; 383: 546-57.
8.
Kappos L, Cohen J, Gold R, et al. Five-Year Efficacy Outcomes of Ofatumumab in Relapsing MS Patients: Insights From ALITHIOS Open-label Extension Study. Poster number: EPR-097. Poster presentation at the European Academy of Neurology (EAN) 2023; July 1-4, 2023; Budapest.
9.
Bar-Or A, Hauser SL, Cohen JA, et al. Early initiation of ofatumumab delays disability progression in people with relapsing MS: 6-year results from ALITHIOS Open-Label Extension Studz. ECTRIMS 2024. P058.
10.
Pardo G, Hauser SL, Bar-Or A, et al. Longer-term (up to 6 years) efficacy of ofatumumab in people with recently diagnosed and treatment-naïve relapsing multiple sclerosis. Oral presentation at the American Academy of Neurology (AAN) 2024 Annual Meeting; April 13-18, 2024; Denver, CO.
11.
Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162-73.
12.
Kragt JJ, Nielsen IM, van der Linden FA et al. How similar are commonly combined criteria for EDSS progression in multiple sclerosis? Mult Scler 2006; 12: 782-6.
13.
U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2017. https://ctep.cancer.gov/protoc....
14.
Gartner J, Hauser SL, Bar-Or A, et al. Benefit-risk of ofatumumab in treatment-naive early relapsing multiple sclerosis patients. J Neurol Neurosurg Psychiatry 2022; 93: A136.2.
15.
Pfeuffer S, Wagner C, Nelles G. Ofatumumab, interferon 1 and glatiramer acetate as first-line treatment in real-world clinical routine: the AIOLOS study. ECTRIMS2024 P1762.
16.
Bischof F, Groth Mand Redolfi J. KAIROS: real world evidence of ofatumumab in patients with relapsing remitting multiple sclerosis who previously received another disease-modifying therapy. ECTRIMS 2024. P1744.
17.
Van der Walt A, Butzkueven H, Spelman T, et al. Real-world Australian experience with ofatumumab in the MSBase Registry. ECTRIMS 2024.P814.
18.
Larsson V, Forsberg L, Hilłert J, et al. Clinical Effectiveness and Safety of Ofatumumab for Patients Treated since March 2021 in the Swedish Post-Market Surveillance Study “Immunomodulation and Multiple Sclerosis Epidemiology 12” (IMSE 12). ECTRIMS 2024 P1696.
19.
Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol 2019; 76: 536-41.
20.
Chisari C, Bucello S, Cottone S, et al. Real-world evidence for ofatumumab in multiple sclerosis: a Sicilian multicenter experience. Eur J Neurol 2024; 31 Suppl 1. Abstracts of the 10th Congress of the European Academy of Neurology. EPO-269.
21.
He A, Merkel B, Brown JWL, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol 2020; 19: 307-16.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.