The hepatitis E virus (HEV) antibody seroprevalence in Europe varies greatly, with hot spots of HEV infection (southwest France, the Netherlands, Scotland, western Germany, Czech Republic, central Italy and several regions of Poland) [1–3]. Food-borne transmission of HEV appears to be a major route of infection in Europe and pigs and wild boars are considered the main animal reservoirs of the virus. Moreover, there have been described asymptomatic blood donors who were viremic at the time of donation, facilitating transmission via blood products [4]. The most common HEV genotype described so far in European countries is genotype 3 (HEV-3). However, a few cases of autochthonous HEV-4 have been reported [1]. In cases of fulminant hepatitis E (HE) with liver failure and in severely immunocompromised patients, treatment with ribavirin is an option [1]. Most of the previous experience with HE comes from Western European countries.
The aim of this study was to describe typical epidemiological and clinical characteristics of patients with hepatitis E in the Czech Republic. We believe that the similar characteristics of the disease can be assumed for the surrounding countries as well, based on numerous similar cultural and sociological aspects.
Methods
We retrospectively retrieved the medical information of patients with HE between June 2012 and October 2021, treated at the Department of Infectious Diseases, University Hospital Brno, Czech Republic and at the Centre of Cardiovascular and Transplant Surgery Brno, Czech Republic. The study was approved by the Ethical Committee of The University Hospital Brno and all patients signed the informed consent form.
Diagnosis was considered as confirmed in patients with positive detection of HEV RNA in the serum or stool (RealStar HEV RT-PCR Kit 1.0, Altona Diagnostics, Germany). Because of HEV RNA clearance from the serum or plasma within an average of 3 weeks after the appearance of symptoms, in cases with negative quantitative reverse transcription polymerase chain reaction (RT-qPCR) test results or RT-qPCR not examined, the serum was tested for the presence of anti-HEV antibodies twice in 1 month. In cases of confirmed positivity of anti-HEV IgM antibodies in the first sample and at least anti-HEV IgG antibodies in the second sample (both ELISA HEV kits, Dia.Pro company, Italy), we included patients in the study group. The possibility of autoimmune hepatitis was ruled out by autoantibody testing and all the patients were serologically tested to exclude hepatitis A, B and C. Anti-HEV IgM positive patients were excluded when the RT-qPCR tested negative and any other viral hepatitis was confirmed or when anti-HEV IgG antibodies were not detected in the second sample or in the case of persistent hepatopathy with another known explanation or in the absence of follow-up. Additionally, the presence of HEV RNA in the stool and cerebrospinal fluid samples (in patients with neurological symptomatology) was detected by RT-qPCR as described by Vasickova et al., 2012 [5]. The subgenomic region (242 nt long sequences within the ORF1 gene) of the detected HEV was subjected to sequencing (Eurofins Genomics). Sequence and phylogenetic analysis was performed by Mega6 software. The obtained HEV sequences were compared to prototype sequences of HEV subtypes proposed by Smith et al. [6] and submitted to GenBank.
The study was approved by the Ethical Committee of the University Hospital Brno and all patients signed the informed consent form.
Results
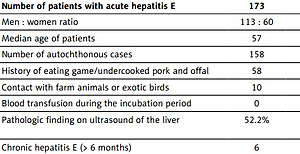
In total, 173 patients with acute HE were assessed in the study. There were 126 patients with RT-qPCR HEV RNA positivity in either the serum or stool and another 47 patients included because of acute hepatitis with anti-HEV IgM antibodies present in the first blood sample and at least IgG antibodies in the second sample.
The majority of patients were men (65.3%). The median age of all patients was 57 years, with a range between 14 and 86 years. None of the patients had been vaccinated against HE prior to infection. During a year, increased incidence of autochthonous cases in the winter and spring was observed. HEV genotyping was successful in 59 patients, revealing infection with HEV-3 in 56 and with HEV-1 in 3 patients with a history of travelling to Sri Lanka and India. HEV-3 sequences showed 95–100% similarity to HEV originating from patients from other Czech regions. A high similarity was observed between the analysed sequences and HEV found in domestic pigs or wild boar as well. A comparison of the obtained HEV-3 sequences with data stored in GenBank revealed significant genetic similarity of HEV-3 sequences to sequences found in European inhabitants (especially Germany, the Netherlands, France and the United Kingdom). Present sequences of the specific part of the HEV genome are available in the GenBank database under the numbers OL802856-OL802909.
The patients’ baseline characteristics are listed in Tables I and II. Only 38 (34%) patients presented with jaundice. In the absence of jaundice, testing for the possibility of HE was generally motivated by flu-like symptoms, including myalgia, arthralgia and low-grade fever, abdominal pain or discomfort or by neurological symptoms with elevated ALT and AST serum activity. Most of the patients with acute HE were admitted to the hospital (n = 112, 64.7%).
Table I
Overview of selected characteristics of patients diagnosed with hepatitis E at the University Hospital Brno
Table II
Median of the maximal values of serum bilirubin concentration and aminotransferase activity in individual patients. Possible imported cases were excluded and asymptomatic cases were excluded because of unknown onset of the disease
| Parameter | Minimum | Maximum | Mean | SD | CoV |
|---|---|---|---|---|---|
| Bilirubin [mg/dl] | 0.3 | 39 | 2 | 8 | 420% |
| ALT [IU/l] | 47 | 8353 | 1129 | 1433 | 127% |
| AST [IU/l] | 27 | 5319 | 638 | 977 | 153% |
| GGT [IU/l] | 32 | 3061 | 351 | 453 | 129% |
| ALP [IU/l] | 50 | 615 | 192 | 117 | 61% |
Nineteen patients experienced decompensation of pre-existing liver disease. Of them, 17 were treated with ribavirin at doses of 600–1200 mg/day. In most of these patients, corticosteroids were used as well. Five patients underwent liver transplants as a consequence of acute-on-chronic liver failure triggered by HE.
Seventeen patients with confirmed HE were assumed to be immunodeficient due to ongoing treatment with immunosuppressive drugs, of whom 5 developed chronic HE defined as the presence of viremia for more than 6 months. In the patient after a heart transplant, the reduction of immunosuppressive therapy (mycophenolate mofetil and tacrolimus) was followed by viral clearance and normalization of serum aminotransferases activity in 7 months, without using ribavirin. The 5 patients after liver or kidney transplant were treated using ribavirin 800–1200 mg/day for 1 to 12 months. In 1 case, two relapses of viremia with hepatitis recurred, so the treatment with ribavirin was reinitiated 3 times over 2 years.
Possible extrahepatic manifestations were noted in 8 patients with acute HE. These cases included uncomplicated meningoencephalitis, meningoencephalitis with a consecutive postencephalitic syndrome, 2 cases with bilateral asymmetric neuralgic amyotrophy, 1 case with concomitant infliction of the phrenic nerve on the same side, a patient with peripheral facial nerve palsy with normal cerebrospinal fluid findings and a patient with tinnitus and vertigo, again without pathological findings in the cerebrospinal fluid. All of these were infected with HEV-3. Two of them were women and 6 were men, with a median age of 46 years, range: 31–63 years. Peak ALT levels in these patients ranged from 41 to 7147 U/l, with bilirubin concentrations from 0.3 to 10.5 mg/dl, while only one developed jaundice. HEV RNA detection in the cerebrospinal fluid was positive only in 1 case of aseptic meningoencephalitis (HEV-3c). Five patients were treated with corticosteroids according to local expertise and one with ribavirin 1000 mg/day for 12 weeks also. Neither intravenous immunoglobulins nor plasmapheresis was used in these cases. The outcome was favourable overall in all of the patients. However, unilateral phrenic nerve paresis and other minor motor deficits persist in the cases of neuralgic amyotrophy, and depression syndrome followed after meningoencephalitis. Some of the neurological manifestations have been reported before [7]. Others are previously unpublished, and a direct connection with HE in these patients has not been peer-reviewed yet.
Discussion
Our study cannot provide any information on the incidence of HE in the Czech Republic as it is a retrospective study on patients undergoing differential diagnosis of acute hepatitis only, and according to our conviction, misses the majority of asymptomatic cases. The only serological survey carried out in the Czech Republic so far found a 6.7% prevalence of anti-HEV IgG antibodies [8].
According to the case history obtained from our patients, undercooked pork meat and less often wild boar meat could probably be the vehicle of HE infections in the Czech Republic. The findings of genetically almost identical human HEV from different parts of the Czech Republic during several years with no direct connection indicate the possibility of virus circulation in the environment. We did not find any case of person-to-person transmission in HEV-3 infected patients.
Infection tends to affect older men, with the sex ratio and median age similar to previously published data from Western Europe. With the 11% incidence of decompensation of pre-existing liver disease in our study, we observed a higher risk compared to Western Europe, where only 11/343 (3.2%) patients with decompensated chronic liver disease followed in France or the UK had acute hepatitis E and three of those died. Exact data on liver failure in hepatitis E patients only are not available. Four of the 173 (2.3%) patients in our series died as a consequence of acute HEV infection. All of them had a history of alcohol abuse, which explained the corticosteroid treatment. Ribavirin treatment was introduced in 17 patients once HEV infection was documented. However, the patients died shortly thereafter. An earlier diagnosis might have allowed the initiation of ribavirin treatment more rapidly and successfully. Thus, testing for HEV infection should be considered in patients with presumed alcoholic hepatitis. In 5 organ transplant cases, ribavirin was used; in 1 case the decrease of the immunosuppression level without ribavirin was sufficient. The treatment protocols followed the experiences of previously reported case studies [9].
There have been several cohort and case studies of neurological injuries in patients with HEV infection [1, 10–12]. In our study, 8 of 173 patients (4.6%) presented with neurological symptoms. Compared to previously published studies, we used corticosteroids more often (5 of 8 patients), with no serious side effects but a favourable outcome. The views on the treatment of neuralgic symptomatology probably related to HE are diverse and there is still no recommended treatment protocol.
Despite the lack of transfusion-related cases in our study, the possibility of transmission of the infection via blood products has been repeatedly demonstrated in many countries [4]. Transfusion product testing for the presence of HEV should be considered at least in immunocompromised recipients, who are at higher risk of developing life-threatening complications of the infection.
In conclusion, testing for HEV should be included early in the diagnostic workup of acute hepatitis, including alcoholic hepatitis, neuralgic amyotrophy, Guillain-Barré syndrome and aseptic meningoencephalitis.