Introduction
Type 2 diabetes mellitus (T2DM) is among the most prevalent diseases globally and is characterized by increased blood glucose levels [1]. The primary pathophysiological mechanism underlying chronic hyperglycemia and T2DM is the endogenous resistance of body cells to insulin [2]. Treatment for T2DM typically involves oral medications, such as biguanides and alpha-glucosidase, and insulin injections are required for refractory T2DM cases [3]. Sodium-glucose cotransporter 2 (SGLT2) inhibitors have become increasingly popular in T2DM treatment because of their ability to reduce hyperglycemia by approximately 0.5% to 1.0%, which is the primary and most crucial mechanism of SGLT2 inhibitors [4, 5]. In another previous study, the combined application of SGLT2 and DPP-4 inhibitors resulted in a 0.71% greater reduction in the glycated hemoglobin level than did DPP-4 inhibitor monotherapy [6].
In addition to having an antihyperglycemic effect, SGLT2 inhibitors exert a protective effect on other areas of the body [4, 7], and SGLT2 inhibitors can reduce the incidence of several diseases [8–11]. SGLT2 inhibitors were discovered to be beneficial for managing cognitive impairment [12]. Furthermore, patients with T2DM using SGLT2 inhibitors were discovered to have a substantially lower incidence of myocardial infarction compared to those not using such inhibitors [8]. SGLT2 inhibitors also exert renoprotective effects, including improving the glomerular filtration rate [9]. Furthermore, population-based studies have indicated that the use of SGLT2 inhibitors can reduce the incidence of eye disorders, such as dry eye disease and diabetic retinopathy [10, 13, 14]. In addition, SGLT2 inhibitors were reported to exert an anti-inflammatory effect by regulating proinflammatory cytokine expression [7]. For example, SGLT2 inhibitors were demonstrated to suppress inflammatory reactions in an experimental model of autoimmune myocarditis [15]. SGLT2 inhibitors were also reported to possess antioxidant properties, as evidenced by a decrease in reactive oxygen species production following the application of SGLT2 inhibitors in an animal diabetic kidney disease model [16]. Moreover, an experimental study indicated that SGLT2 inhibitors reduced oxidative stress and myocardial fibrosis [17].
Ovarian cancer is the second most common gynecological cancer and has a 5-year survival rate of 47.4% [18]. The known risk factors for ovarian cancer include delayed childbearing, early menarche, a family history of ovarian cancer, and preexisting endometriosis [19]. Ovarian cancer is more likely to develop in individuals with hyperglycemia and T2DM [20], and the mortality rate of ovarian cancer is considerably higher in the T2DM population [21]. The development of ovarian cancer is associated with inflammation, as evidenced by increased levels of interleukins and tumor necrosis factor in patients with ovarian cancer [22]. Furthermore, oxidative stress is strongly associated with the pathogenesis and neoangiogenesis of ovarian cancer [23]. A higher reactive oxygen species level is correlated with metastasis and therapy resistance in ovarian cancer [24]. Still, few studies have evaluated the correlation between the use of SGLT2 inhibitors and the incidence of ovarian cancer, despite SGLT2 inhibitors being able to suppress inflammation, which is a key mechanism in the development of ovarian cancer [16, 22].
The present study investigated the potential protective effect of SGLT2 inhibitors on the development of ovarian cancer by using data from Taiwan’s National Health Insurance Research Database (NHIRD). In addition, this study analyzed the effects of SGLT2 inhibitors on patients with T2DM with different characteristics.
Material and methods
Data source
The current study was conducted in accordance with the Declaration of Helsinki (1964) and its later amendments. The study was approved by Chung Shan Medical University Hospital. The requirement for written informed consent was waived by both institutions. The NHIRD contains claims data for 23 million Taiwanese individuals, with the data covering the period from January 1, 2000, to December 31, 2021.
Patient selection
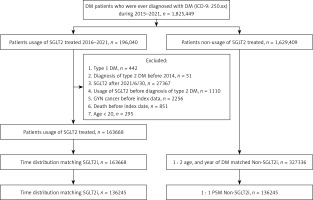
This was a retrospective cohort study. Patients with T2DM using SGLT2 inhibitors who met the following criteria were selected: (1) having a diagnosis of T2DM based on ICD-9-CM or ICD-10-CM codes between 2015 and 2021; (2) visiting an internal or a family medicine physician for more than 3 months; (3) undergoing a glycated hemoglobin examination prior to the T2DM diagnosis; and (4) receiving a prescription for SGLT2 inhibitors, such as dapagliflozin, canagliflozin, empagliflozin, or ertugliflozin, as identified using ATC codes. The index date for this study was defined as 6 months after the initial SGLT2 inhibitor prescription. The following exclusion criteria were applied to standardize the T2DM population: (1) absence of demographic data, (2) use of antidiabetic medication before the T2DM diagnosis, (3) age younger than 20 years or older than 100 years, and (4) diagnosis of any gynecological cancer before the index date. Propensity score matching (PSM) was used to match the SGLT2 inhibitor group with a control group, with demographics, systemic covariates, and associated medications considered. After PSM, 136 245 patients with T2DM were included in both the SGLT2 inhibitor group and the control group. Figure 1 presents the flowchart of patient selection.
Main outcome
The primary outcome in this study was the development of ovarian cancer, defined on the basis of the following criteria: (1) receiving a diagnosis of ovarian cancer based on ICD-9-CM or ICD-10-CM diagnostic codes; (2) receiving a pelvic examination before or at the time of the ovarian cancer diagnosis, as indicated by procedure codes; (3) undergoing a computed tomography scan, pelvic ultrasound examination, or cancer antigen 125 (CA-125) test before or at the time of the ovarian cancer diagnosis, as indicated by procedure codes; and (4) receiving a diagnosis confirmed by a gynecologist. Included patients with T2DM were followed until one of the following events occurred: (1) a diagnosis of ovarian cancer, (2) withdrawal from the NHI program, or (3) the end of the study period on December 31, 2021, as recorded in Taiwan’s NHIRD.
Associated confounders
In addition to ovarian cancer events, we considered several demographic factors and systemic disorders in our analysis and adjusted for their potential confounding effects on ovarian cancer development. These included age, urbanization level, hypertension, coronary heart disease, hyperlipidemia, cerebrovascular disease, peripheral vascular disease, endometriosis, ovarian cysts, and chronic kidney disease, with all diseases and conditions identified using ICD-9-CM and ICD-10-CM diagnostic codes. In addition, the analysis accounted for the use of certain T2DM medications, such as biguanides, sulfonylureas, alpha-glucosidase inhibitors, thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors, insulin, and statins, as identified using ATC codes. To ensure that the duration of systemic disorders and medication use was sufficient to determine whether they affected the likelihood of ovarian cancer development, only systemic diseases and medications that persisted or were prescribed for more than 2 years before the index date were included in the analyses.
Statistical analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Descriptive analyses were conducted to examine the distributions of demographic factors and systemic diseases between the 2 groups. The absolute standardized difference (ASD) was used to evaluate differences between the SGLT2 inhibitor and control groups. Subsequently, Cox proportional hazard regression was performed to calculate the adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) for ovarian cancer incidence, comparing the SGLT2 inhibitor and control groups, after adjustment for all demographic factors, systemic diseases, and medication usage at baseline in the regression model. An interaction test was conducted to compare the effect of SGLT2 inhibitors on ovarian cancer development between the groups. A p- value of < 0.05 was considered significant, and p- values less than 0.0001 were reported as p < 0.0001.
Results
Table I lists the baseline characteristics of the SGLT2 inhibitor and control groups. The age distribution was similar between the groups (ASD = 0.0679). In addition, the prevalence of systemic diseases was comparable between the groups (all ASD < 0.1). The use of sulfonylureas, α-glucosidase inhibitors, thiazolidinediones, and insulin was also similar between the SGLT2 inhibitor and control groups (all ASD < 0.1;Table I).
Table I
Baseline characteristics of SGLT-2 inhibitor users and matched comparison
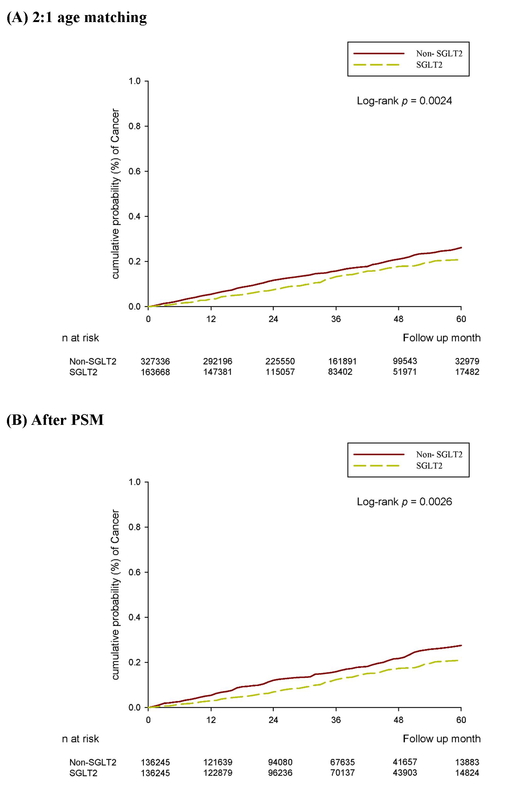
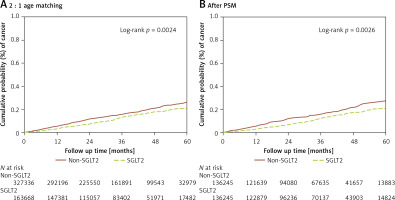
Over the follow-up period of up to 6 years, 205 and 515 cases of ovarian cancer were observed in the SGLT2 inhibitor and control groups, respectively, after age matching at a ratio of 2 : 1 (Table II). Furthermore, 167 and 222 cases of ovarian cancer were identified in the SGLT2 inhibitor and control groups, respectively, by using the PSM method (Table II). The incidence of ovarian cancer was significantly lower in the SGLT2 inhibitor group than in the control group in the multivariable analysis (aHR = 0.73, 95% CI: 0.60–0.89, p = 0.0023; Table III). Moreover, the cumulative probability of ovarian cancer was lower in the SGLT2 inhibitor group than in the control group after PSM (p = 0.0026; Figure 2). In the subgroup analysis stratified by different oral medications, 198 and 313 cases of ovarian cancer were noted in the SGLT2 inhibitor and biguanide groups, respectively (Table IV). The incidence of ovarian cancer was significantly lower in the SGLT2 inhibitor group than in the biguanide group (aHR = 0.74, 95% CI: 0.62–0.90; Table IV). In addition, the effects of SGLT2 inhibitors on ovarian cancer development significantly differed between the sulfonylurea, α-glucosidase inhibitor, and DPP4 inhibitor groups (all p < 0.05; Table IV).
Table II
Incidence rate of ovarian cancer between SGLT-2 inhibitor and control groups
| Variable | 2 : 1 age matching | After PSM | ||||
|---|---|---|---|---|---|---|
| Non-SGLT2 | SGLT2 | P-value | Non- SGLT2 | SGLT2 | P-value | |
| N | 327336 | 163668 | 136245 | 136245 | ||
| Follow-up person-months | 11191912 | 5706341 | 4668860 | 4777068 | ||
| New cases | 515 | 205 | 222 | 167 | ||
| Incidence rate*(95% C.I.) | 0.46 (0.42-0.50) | 0.36 (0.31–0.41) | 0.48 (0.42–0.54) | 0.35 (0.30–0.41) | ||
| Crude relative risk (95% C.I.) | Reference | 0.78 (0.66–0.92) | 0.0027 | Reference | 0.74 (0.60–0.90) | 0.0026 |
| Adjusted HR* (95% C.I.)† | Reference | 0.71 (0.59–0.85) | 0.0002 | Reference | 0.73 (0.60–0.89) | 0.0023 |
Table III
Multiple Cox regression to estimate the hazard ratio of ovarian cancer
Table IV
Incidence rate of ovarian cancer by oral medication cases versus SGLT2 inhibitor cases
| Variable | 2 : 1 age matching | |
|---|---|---|
| Biguanides | SGLT2 | |
| N | 179428 | 149456 |
| Follow-up person-months | 6351636 | 5262944 |
| New cases | 313 | 198 |
| Incidence rate*(95% C.I.) | 0.49 (0.44–0.55) | 0.38 (0.33–0.43) |
| Crude relative risk (95% C.I.) | Reference | 0.76 (0.64–0.91) |
| Adjusted HR* (95% C.I.)† | Reference | 0.74 (0.62–0.90) |
| Sulfonylureas | SGLT2 | |
| N | 73873 | 66089 |
| Follow-up person-months | 2701778 | 2347071 |
| New cases | 139 | 79 |
| Incidence rate*(95% C.I.) | 0.51 (0.44-0.61) | 0.34 (0.27–0.42) |
| Crude relative risk (95% C.I.) | Reference | 0.65 (0.50–0.86) |
| Adjusted HR* (95% C.I.)† | Reference | 0.66 (0.49–0.88) |
| α-glucosidase inhibitors | SGLT2 | |
| N | 22845 | 27730 |
| Follow-up person-months | 826215 | 1042580 |
| New cases | 36 | 26 |
| Incidence rate*(95% C.I.) | 0.44 (0.31-0.60) | 0.25 (0.17–0.37) |
| Crude relative risk (95% C.I.) | Reference | 0.57 (0.35–0.95) |
| Adjusted HR* (95% C.I.)† | Reference | 0.51 (0.30–0.87) |
| DPP4 | SGLT2 | |
| N | 54086 | 62791 |
| Follow-up person-months | 1799628 | 2225113 |
| New cases | 99 | 82 |
| Incidence rate*(95% C.I.) | 0.55 (0.45–0.67) | 0.37 (0.30–0.46) |
| Crude relative risk (95% C.I.) | Reference | 0.67 (0.50–0.90) |
| Adjusted HR* (95% C.I.)† | Reference | 0.65 (0.48–0.88) |
Discussion
In the present study, the use of SGLT2 inhibitors in patients with T2DM was correlated with a lower incidence of ovarian cancer. In previous studies, SGLT2 inhibitor use was associated with decreased growth of certain cancer cells, such as breast and liver cancer cells [25, 26]. However, no studies have explored the correlation between SGLT2 inhibitor use and gynecological cancer development. To the best of our knowledge, this is the first study to demonstrate the protective effect of SGLT2 inhibitors on the development of ovarian cancer in the T2DM population. A clear temporal relationship between SGLT2 inhibitor use and ovarian cancer development was established by excluding patients who developed ovarian cancer within 6 months prior to initiating SGLT2 inhibitor treatment. In addition, several risk factors, including age and endometriosis, were included in the Cox proportional hazard regression to adjust for their potential effects on ovarian cancer development [19, 27]. Thus, the use of SGLT2 inhibitors is an independent protective factor for ovarian cancer. Given that SGLT2 inhibitors possess multiple functions that can counteract the mechanisms underlying ovarian cancer development [24, 28–30], their use in patients with T2DM may reduce their risk of ovarian cancer. This hypothesis is supported by the findings of the current study. On the other hand, the SGLT2 inhibitors can promote the cell cycle arrest and the apoptotic cell death of colorectal cancer cells [31]. In research discussing the potential for treatment of cancer with an SGLT2 inhibitor, the use of an SGLT2 inhibitor can increase the responsiveness of prostate cancer to radiotherapy [32], and the SGLT2 inhibitor could serve as management for breast cancer, whether as monotherapy or in combination with other agents [33]. Furthermore, the application of SGLT2 inhibitors was found to correlate with lower mortality risk of breast cancer and significantly reduced risk of prostate cancer in recent epidemiological studies [34, 35], which supported the potential for application of SGLT2 inhibitors as cancer treatment. Consequently, the protective effect of the SGLT2 inhibitor on ovarian cancer development in this study could be a definitive effect rather than an incidental finding. In this study, ovarian cancer occurred in 0.12% of the patients with T2DM using SGLT2 inhibitors and 0.16% of those not using SGLT2 inhibitors. The incidence of ovarian cancer in both the SGLT2 inhibitor and control groups in the present study was lower than that reported in a previous study [36]. This difference may be attributable to the effect of hyperglycemia on ovarian cancer development [20].
From an epidemiological perspective, T2DM is a prevalent disease affecting approximately 10% of the global population [3]. Furthermore, the incidence of T2DM is rising, with 700 million individuals expected to be affected by 2040 [2]. SGLT2 inhibitors, which are effective antihyperglycemic medications, are widely used in the treatment of T2DM [3, 37]. In the United States, approximately 11.2% of patients with T2DM use SGLT2 inhibitors for glycemic control [38]. In addition, ovarian cancer is the second most common gynecological cancer worldwide, after breast cancer [18]. In 2018, 240 000 individuals were given a diagnosis of ovarian cancer [18], and approximately 140 000 women die from ovarian cancer globally every year, which poses a substantial socioeconomic burden [39]. Because both T2DM and ovarian cancer affect a large proportion of the population [2, 19], exploring any potential correlations between these 2 diseases, including their management, is crucial.
This study has several limitations. First, a claims database rather than actual medical records was used for analysis. Thus, several crucial data points could not be investigated, including blood sugar and glycated hemoglobin levels in patients with T2DM, trends in these levels, medication adherence to SGLT2 inhibitors, the location and pathological details of ovarian cancer, imaging results, treatment response, recurrence of ovarian cancer, and details regarding other systemic diseases. The absent of the above crucial data could reduce the integrity and credibility of our analyses and results significantly. Second, the diagnosis of ovarian cancer was only based on the claimed codes in the NHIRD without the confirmation in histopathology; therefore the accuracy of ovarian cancer diagnosis in this study may be doubted. With the solitary usage of ICD-9-CM (183.0) or ICD-10-CM (C56) codes as diagnostic criteria, whether the corresponded lesion was invasive ovarian cancer or borderline ovarian tumors cannot be confirmed. Due to the design of the Taiwan NHIRD, we can only trace one exposure-to-outcome event (i.e. SGLT2 inhibitor to ovarian cancer) in one cohort study; hence the survival data since the ovarian cancer diagnosis cannot be traced in this study. Also, smoking and obesity are known risk factors for ovarian cancer [19], but these factors are rarely documented by physicians in the claims database. Thus, we were unable to adjust for their effects in the Cox proportional hazard regression analysis. In addition, although we employed PSM to improve the comparability between the SGLT2 inhibitor and control groups, the retrospective nature of the current study may have reduced the homogeneity of the study population relative to that achievable with a prospective study. Finally, nearly all participants in the current study were Taiwanese, which may limit the external validity of the current findings.
In conclusion, this preliminary study showed that the use of SGLT2 inhibitors is associated with a lower incidence of ovarian cancer in the T2DM population, even after adjustment for several confounders. Thus, the administration of SGLT2 inhibitors can be recommended for patients with T2DM who have known risk factors for ovarian cancer. However, additional large-scale prospective studies should be conducted to evaluate the correlation between SGLT2 inhibitor use and the treatment response of ovarian cancer.