Introduction
Lower limb varicose veins (VVs) are the most common vascular condition and may cause hyperpigmentation, eczema, and active ulcers, resulting in a significant decrease in quality of life (QoL) [1]. The therapeutic procedures for VVs include conventional surgery, endovenous thermal ablation, foam sclerotherapy, and mechanochemical ablation, whereas endovenous laser ablation (EVLA) and radiofrequency ablation (RFA) are the most widely used and guideline-recommended procedures (IB recommendations) [2–10]. EVLA and RFA procedures typically involve thermal ablation of the great saphenous vein (GSV) above the knee or foam sclerotherapy and pin stripping for VVs below the knee. Although relatively satisfactory outcomes have been achieved with these procedures compared with traditional surgery, due to the technical defects of sclerotherapy for VVs, routine thermal ablation procedures may result in a higher rate of VV recurrence below the knee, which may affect the long-term outcomes and QoL of patients [11–13]. In addition, different centres have also reported different results regarding the effect of endovenous thermal ablation [14–17]. Therefore, exploring a more effective ablation procedure for VVs may be crucial to reduce recurrence; however, studies on this topic are limited.
Many experts have tested different endovenous strategies to improve the outcomes of treating VVs below the knee. Previous reports have indicated that patients with VVs below the knee can undergo regular follow-up; alternatively, a staged therapeutic strategy or a hybrid procedure can be employed [18, 19]. A previous study reported that 91% of patients display persistent reflux below the knee and that the clinical signs and symptoms become worse after surgery [20]; thus, some experts believe that varices below the knee should be treated actively [21] while other experts believe that varices below the knee should not be treated immediately [22]. Therefore, the optimal therapeutic strategy for varices and trunk reflux below the knee remains controversial. However, it is clear that staged therapy may cause increased patient anxiety and treatment costs [23, 24]. Therefore, hybrid procedures, including thermal ablation, sclerotherapy, and pin stripping, may be more in line with patient treatment demands and reduce the total treatment cost. In this study, we retrospectively compared the outcomes of hybrid EVLA and RFA procedures for VVs and analysed the VV recurrence rate and patient QoL.
Material and methods
Patients
Between July 2019 and December 2020, 192 patients with VVs from our university-affiliated hospital were included in this retrospective study. The patients were divided into 2 groups according to the treatment they received (the thermal ablation used was determined according to the willingness of the patient or guardian). In total, 84 patients (121 limbs) were included in the EVLA group, and 108 patients (151 limbs) were included in the RFA group. All patients underwent preoperative ultrasonography by professional physicians, and patients with iliac vein compression syndrome were excluded based on antegrade venography or computed tomography venography (CTV) results. This study was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University, and all patients signed informed consent forms.
The inclusion criteria for this study were as follows: (1) symptomatic VVs classified as C2–C6 according to the revised Clinical-Etiology-Anatomy-Pathophysiology (CEAP) classification [25]; (2) GSV reflux time > 0.5 s; (3) age from 18 to 75 years; and (4) physician experience of > 300 ablation procedures. The exclusion criteria were as follows: (1) severe cardiopulmonary insufficiency, coagulation disorder and other surgical contraindications or inability to tolerate the procedure; (2) history of deep vein thrombosis (DVT); (3) history of VV procedures in the ipsilateral limb; (4) iliac vein compression syndrome; (5) serious infection at the treatment site; (6) GSV diameter > 14 mm; and (7) arterial occlusion or stenotic lesions.
Endovenous procedure
All VV lesions, perforator veins, and venous reflux points were marked under ultrasound guidance in the standing position before the procedure. The procedures were performed under local tumescent anaesthesia. Additional GSV high ligation was performed for patients with a GSV diameter greater than > 10 mm.
Thermal ablation
The EVLA and RFA procedures were performed as previously reported [7]. Briefly, the GSV was cannulated just below the knee under ultrasound guidance, and then a radial laser fibre (1470 nm, Eufoton, Italy) was inserted 2 cm below the saphenofemoral junction. Laser ablation (power: 10 watts, energy: 70 J/cm) was performed under ultrasound guidance. The same technique was used in the RFA group; the vein was ablated at a temperature of 120°C for 20 s per segment using a radiofrequency catheter (ClosureFast, Medtronic, USA).
The procedure for VVs below the knee was as follows: In the EVLA group, laser ablation (bare fibre, power: 10 W) was performed for VVs with a diameter ≥ 5 mm under ultrasound guidance, and the tortuous and dilated varices were treated using ultrasound-guided foam sclerotherapy and a pin stripping (mini-phlebectomy) procedure; sclerosant (polidocanol, Aethoxysklerol; Kreussler, Germany) foam was prepared by using the Tessari method (sclerosant-to-air ratio of 1 : 4) [26, 27]. In the RFA group, the tortuous and dilated varices were treated with ultrasound-guided foam sclerotherapy and pin stripping (mini-phlebectomy) only.
All patients underwent eccentric compression bandaging with elastic bandages for 24 h and were instructed to ambulate as soon as possible postoperatively. After the bandages were removed, all patients were recommended to wear gradient compression stockings (25 mm Hg, ankle, only day wear) for at least 4 weeks.
Evaluation and follow-up
Parameters related to the procedures, including the operation time (from the beginning of the operation to the completion of bandaging), number of limbs treated with high ligation, number of incisions, estimated intraoperative bleeding, hospital stay, sclerosant dosage and 24-hour postoperative pain score, were recorded for all patients in the 2 groups. Pain was evaluated using a visual analogue scale (VAS), ranging from 0 to 10, with greater scores indicating more severe pain.
Complications occurring after the procedures, such as incisional haematoma, DVT, incisional infection, ecchymosis, burns, induration, and numbness, were recorded. Bruising affecting more than 2 cm2 of the skin in the treated area at 48 h after the procedure was defined as ecchymosis. According to the criteria for burns, skin damage, such as red and oedematous areas, observed 48 h after the procedure was defined as skin burns; the presence of sensory disturbances in the skin (numbness) was defined as numbness [28–30]. All patients attended follow-up visits at 1, 6, and 12 months after the procedure. The GSV closure rate was detected by ultrasound examination at 12 months in the 2 groups, and recanalization was defined as an open refluxing segment of the GSV greater than 5 cm in length [7]. Clinical recurrence was defined by clinical examination and patient symptoms. New varices that appeared in the ablated area or a different area that was not detected before the procedure were defined as recurrence [28].
Patient symptom relief was assessed using the Venous Clinical Severity Score (VCSS), and the improvement in QoL was assessed using the Chronic Venous Disease QoL questionnaire (CIVIQ-20) [29–32], both of which are widely used all over the world. Patients in both groups independently completed the VCSS and CIVIQ-20 questionnaires at admission and 1, 6, and 12 months after the procedure. The VCSS score is based on 10 items; each item is scored from 0 to 3 points, for a total possible score of 30 points, and higher scores indicate more severe symptoms. The CIVIQ-20 scoring system includes 4 dimensions (physical, psychological, social, and pain); the total score ranges from 0 to 100, and a higher score indicates poorer QoL. Patient satisfaction was evaluated according to each patient’s self-reported numerical responses, with 0 representing the lowest satisfaction score and 10 representing the highest satisfaction score.
Ethical approval and consent to participate
This study complied with the Declaration of Helsinki Principles and was approved by the Institutional Ethical Committee of the First Affiliated Hospital of Xi’an Jiaotong University.
Statistical analysis
All procedure-related data and follow-up data were collected in an Excel file (v 2013; Microsoft, Redmond, WA, USA), and SPSS 21.0 software (SPSS, Chicago, IL) was used for statistical analysis. Categorical data for the 2 procedures are presented as n (%) and were analysed using the χ2 test. Continuous measurement data were first tested for normality, and normally distributed data are described as the mean (standard deviation). Hypothesis significance testing was performed using the 2-sample t test (2-tailed). Kaplan-Meier survival analysis was used to analyse the recurrence of VVs, and the odds ratio (OR) and 95% confidence interval (95% CI) were calculated. P < 0.05 was considered to indicate a statistically significant difference.
Results
Demographic characteristics and baseline data
The demographic characteristics and baseline data of the 2 groups are presented in Table I. Although the proportion of male patients in the EVLA group was greater than that in the RFA group (63.10% vs. 43.52%, p = 0.02), no differences in other baseline data were noted. No significant difference in the prevalence of bilateral VVs in the lower limbs was noted between the 2 groups, and deep vein reflux was present in 66.12% and 55.63% of limbs in the EVLA and RFA groups, respectively. The GSV diameters at the origin, knee, and ankle were similar in the EVLA and RFA groups.
Table I
Demographic and baseline data for patients in the EVLA and RFA groups
| Parameter | EVLA (n = 84) | RFA (n = 108) | P-value* |
|---|---|---|---|
| Sex (M) | 53 (63.10) | 47 (43.52) | 0.02 |
| Age [years] | 54.33 ±11.12 | 55.88 ±11.10 | 0.34 |
| BMI [kg/m2] | 24.48 ±3.24 | 24.97 ±3.65 | 0.33 |
| Affected limb: | 0.69 | ||
| Left | 31 (36.90) | 39 (36.11) | |
| Right | 16 (19.05) | 26 (24.07) | |
| Bilateral | 37 (44.05) | 43 (39.82) | |
| CEAP: | 0.40** | ||
| C2 | 25 (20.66) | 26 (17.22) | |
| C3 | 53 (43.80) | 54 (35.76) | |
| C4 | 30 (24.79) | 53 (35.10) | |
| C5 | 4 (3.31) | 6 (3.97) | |
| C6 | 9 (7.44) | 12 (7.95) | |
| Deep vein reflux (Y) | 80 (66.12) | 84 (55.63) | 0.21** |
| GSV diameter [mm]: | |||
| Origin | 8.23 ±1.89 | 7.75 ±2.05 | 0.71** |
| Knee | 4.64 ±1.28 | 4.85 ±0.93 | 0.71** |
| Ankle | 2.18 ±0.39 | 2.11 ±0.29 | 0.71** |
| Smoking | 53 (63.10) | 56 (51.85) | 0.08 |
| Comorbidities: | 26 (30.95) | 38 (35.19) | 0.53 |
| Diabetes | 1 (1.19) | 2 (1.85) | |
| Hypertension | 11 (13.10) | 18 (16.67) | |
| CAD | 5 (5.95) | 4 (3.71) | |
| Tumour history | 2 (2.38) | 5 (4.63) | |
| Other | 7 (8.33) | 9 (8.33) |
Procedure-related data
The success rate of the procedure was 100% in both groups, and the procedure-related data are shown in Table II. There was no significant difference in the number of limbs that underwent high ligation (14.05% vs. 14.57%) or the number of pin stripping incisions (2.34 ±1.12 vs. 2.21 ±1.02) between the EVLA and RFA groups. The operation time, estimated bleeding, postoperative hospital stay, and 24-hour pain VAS score were similar for both procedures. However, a lower dosage of foam sclerosant was used in the EVLA procedure than in the RFA procedure (12.33 ±4.76 ml vs. 13.98 ±4.92 ml, p = 0.02).
Table II
Procedure-related outcomes for the EVLA and RFA procedures
| Parameter | EVLA (n = 84) | RFA (n = 108) | P-value* |
|---|---|---|---|
| Success rate | 84 (100) | 108 (100) | NA |
| High ligation in limb | 17 (14.05) | 22 (14.57) | 0.90 |
| Operation time [min] | 49.43 ±10.74 | 47.82 ±10.66 | 0.30 |
| Number of incisions | 2.34 ±1.12 | 2.21 ±1.02 | 0.33** |
| Hospital stay [days] | 1.50 ±0.63 | 1.37 ±0.52 | 0.12 |
| Dosage of sclerosant [ml] | 12.33 ±4.76 | 13.98 ±4.92 | 0.02** |
| Estimated bleeding [ml] | 29.22 ±13.74 | 26.24 ±10.86 | 0.11** |
| VAS score at 24 h | 4.31 ±1.38 | 4.19 ±1.23 | 0.51 |
Complications
The complications in the 2 groups are listed in Table III. One (0.66%) patient suffered from DVT at 1 month after the RFA procedure and recovered after 3 months of anticoagulation therapy. The incidences of induration (30.58% vs. 23.18%) and numbness (10.74% vs. 5.96%) in the EVLA group were similar to those in the RFA group. Mild numbness was reported in 2 limbs (1.65%) subjected to the EVLA procedure and in one limb (0.66%) subjected to the RFA procedure at 12 months. No cases of myocardial infarction, stroke or death occurred in either group within 30 days. During the follow-up period, 2 patients in the RFA group died of cardiovascular accidents, and no difference in all-cause mortality was noted between the 2 groups (0% vs. 1.85%, p > 0.05).
Table III
Complications noted after the EVLA and RFA procedures
| Parameter | EVLA (n = 121) | RFA (n = 151) | P-value* |
|---|---|---|---|
| Haematoma | 0 (0) | 0 (0) | NA |
| DVT | 0 (0) | 1 (0.66) | 0.91** |
| Incisional infection | 1 (0.83) | 1 (0.66) | 0.38** |
| Ecchymosis | 32 (26.45) | 46 (30.46) | 0.47** |
| Skin burns | 1 (0.83) | 1 (0.66) | 0.38** |
| Induration | 37 (30.58) | 35 (23.18) | 0.14** |
| Numbness: | |||
| 1 month | 13 (10.74) | 9 (5.96) | 0.15** |
| 6 months | 5 (4.13) | 3 (1.99) | 0.16** |
| 12 months | 2 (1.65) | 1 (0.66) | 0.17** |
| Stroke | 0 (0) | 0 (0) | NA |
| Procedure-related death | 0 (0) | 0 (0) | NA |
Recurrence and QoL
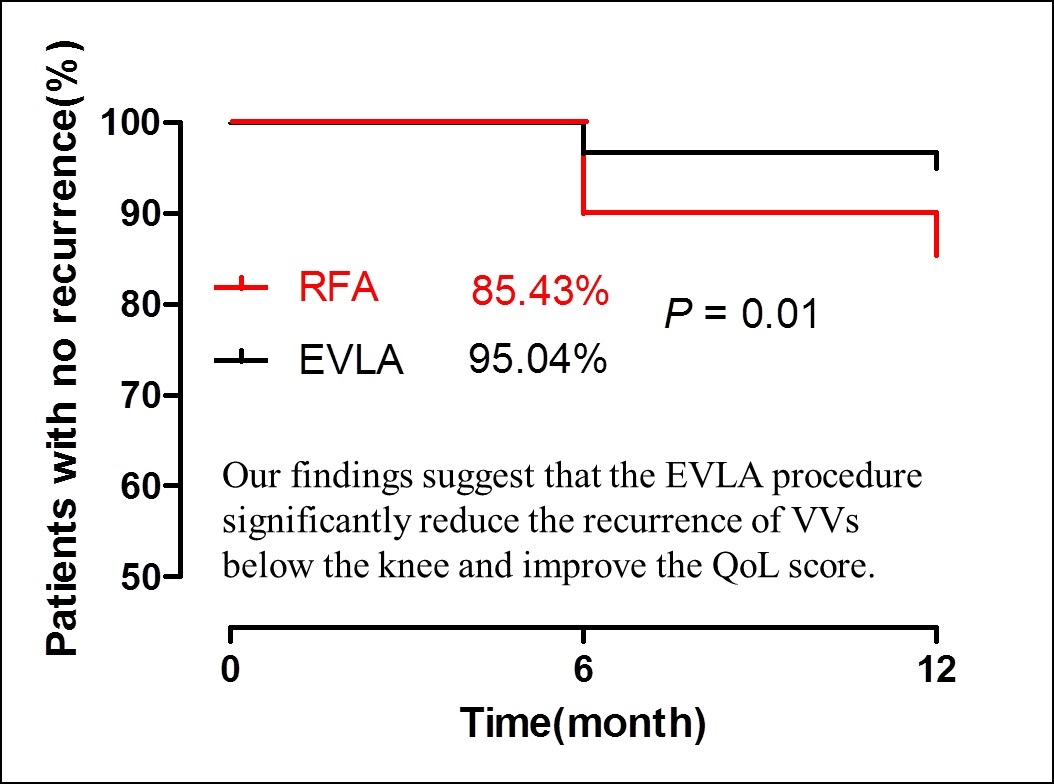
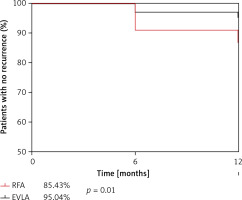
The follow-up results of the 2 groups are shown in Table IV and Figure 1. The EVLA and RFA procedures showed similar closure rates at 12 months (99.2% vs. 97.4%). The VCSS and CIVIQ-20 scores of patients after the procedure were significantly lower than those before the procedure (p < 0.01), with no difference between the procedures at 1 month. However, lower VCSS and CIVIQ-20 scores were reported in the EVLA group than in the RFA group at 6 months and 12 months. The recurrence rate after EVLA was lower than that after RFA (4.96% vs. 14.57%, p = 0.01; OR = 3.27; 95% CI: 1.33–8.00; Figure 1) at 12 months, and VV recurrence in the calf was significantly lower after EVLA than RFA (4.13% vs. 11.92%, p = 0.02; OR = 3.14, 95% CI: 1.18–8.35); however, there was no significant difference in VV recurrence in the thigh between the 2 procedures (0.83% vs. 2.64%, p > 0.05; OR = 3.26, 95% CI: 0.41–26.29). Although patients undergoing both procedures reported higher satisfaction scores at 12 months, the satisfaction scores of those who underwent the EVLA procedure were higher than those who underwent the RFA procedure (9.70 ±0.68 vs. 9.19 ±1.79, p = 0.02).
Table IV
Recurrence and QoL data for the EVLA and RFA procedures
| Parameter | EVLA (n = 121) | RFA (n = 151) | P-value |
|---|---|---|---|
| GSV closure rate | 120 (99.17) | 147 (97.35) | 0.26** |
| VCSS score*: | |||
| Baseline | 6.1 ±3.09 | 6.64 ±3.31 | 0.25 |
| 1 month | 3.07 ±1.96 | 3.29 ±1.41 | 0.38 |
| 6 months | 1.77 ±1.43 | 2.25 ±1.26 | 0.02 |
| 12 months | 1.31 ±1.25 | 1.81 ±1.27 | 0.01 |
| CIVIQ-20 score*: | |||
| Baseline | 48.51 ±11.94 | 49.64 ±12.67 | 0.53 |
| 1 month | 33.23 ±10.69 | 35.47 ±9.34 | 0.12 |
| 6 months | 29.58 ±9.30 | 32.42 ±8.40 | 0.03 |
| 12 months | 27.81 ±8.59 | 30.67 ±8.41 | 0.02 |
| Recurrence**: | |||
| 12 months | 6 (4.96) | 22 (14.57) | < 0.01 |
| Thigh | 1 (0.83) | 4 (2.65) | 0.51 |
| Calf | 5 (4.13) | 18 (11.92) | 0.02 |
| Satisfaction score | 9.7 ±0.68 | 9.19 ±1.79 | 0.02 |
Figure 1
A lower VV recurrence rate was noted for the hybrid EVLA procedure than for the RFA procedure at 12 months, and the hybrid EVLA procedure was associated with a higher percentage of patients with no recurrence (EVLA: 95.04% vs. RFA: 85.41%, p = 0.01, Kaplan–Meier analysis). *p < 0.05, compared with RFA
Discussion
To date, there have been many reports on the short- and long-term effects of RFA and EVLA procedures [15–23], but studies comparing the outcomes of the 2 procedures have reached different conclusions. El Kilic et al. found that RFA was better than EVLA in terms of the GSV closure rate at 3 and 5 years [14]. Eroglu et al. reported no difference between RFA and EVLA in terms of the closure rate or complications [15]. Bozoglan et al. performed RFA and EVLA on different limbs of the same patient and found that EVLA was superior to RFA in terms of the occlusion rate and patient satisfaction [16]. We hypothesize that these differences may be related to the different methods used to treat VVs below the knee because VVs mainly occur in areas below the knee rather than above the knee; therefore, the different procedures performed on VVs below the knee may affect the outcome. Theivacumar et al. found that the secondary intervention rate exceeded 60% after EVLA (810 nm) was exclusively performed. Even when GSV laser ablation was performed above the knee and foam sclerotherapy was performed for VVs below the knee, the secondary intervention rate was as high as 36%, whereas the secondary intervention rate after EVLA for the ablation of all VVs still reached 17% [12]. Our results indicate that recurrence and patient satisfaction are significantly better when using the hybrid EVLA procedure, foam sclerotherapy, and pin stripping for VVs below the knee; however, the GSV closure rate and recurrence rate for VVs in the thigh are similar for the EVLA and RFA procedures. Moreover, staged therapy may not meet the demands of most patients given the increased frequency and costs of therapy and reduced patient satisfaction. Our results confirm that when used exclusively, the hybrid EVLA procedure could reduce recurrence and improve patient satisfaction at 12 months.
Recurrence is the most important indicator for evaluating the long-term effect of VVs; however, studies on recurrence in different areas after the procedure are limited. Furthermore, recurrence below the knee is the most common type of recurrence and is the main source of residual symptoms [11]. Thus, reducing recurrence below the knee should be the main target of endovenous therapy. Lomazzi et al. believed that the main factors affecting recurrence and recanalization after thermal ablation included higher BMI and larger diameter but did not further discuss recurrence in the calf [33]. Gifford et al. confirmed that VVs below the knee can be safely treated by ablation procedures [34]. Yoon et al. suggested that the recurrence rate is lower after EVLA than RFA for calf VVs [35]. Our data also confirmed that the recurrence rate of the hybrid EVLA procedure was lower than that of the RFA procedure. Considering the potential for nerve injury associated with thermal ablation therapy [36], we only used an intermittent EVLA procedure for VVs with a diameter greater than 5 mm below the knee. This hybrid procedure could improve clinical outcomes without increasing temperature-related complications. Our results confirm the efficacy and safety of this hybrid procedure and are also consistent with those a previous report [37]. In addition, this hybrid procedure could significantly reduce the amount of foam sclerosant needed, which also helps to reduce the potential complications associated with the use of this type of agent.
Many experts consider thermal ablation of the GSV to be sufficient when the trunk is less than 10 mm in diameter; however, greater GSV diameters may affect the closure rate and increase recurrence [33]. A previous study confirmed that patients with a GSV diameter greater than 10 mm exhibited a 12-month trunk recurrence rate of 3.2% after high ligation, which was lower than the rates noted for EVLA and RFA [38]. Allegra et al. found that 13% of patients experienced recurrence due to recanalization of the GSV at 5 years [39]. Flessenkämper et al. suggested that EVLA combined with high ligation is associated with less venous reflux at 2 years than traditional surgery and EVLA alone [40]. Therefore, we believe that high ligation in patients with GSV trunk diameters greater than 10 mm is very important to reduce trunk recanalization and recurrence after thermal ablation. Our study indicates that the 12-month trunk recanalization rate for EVLA (0.8%) and RFA (2.6%) was lower after high ligation of affected limbs with a GSV diameter > 10 mm.
The thermal ablation procedure for VVs can relieve symptoms and improve the QoL of patients, and previous studies have also confirmed a decrease in VCSS and CIVIQ-20 scores in patients after thermal ablation [30, 32]. Huang et al. [41] confirmed that the QoL scores can be significantly improved after EVLA and RFA, and another report demonstrated that thermal ablation alone may lead to a higher recurrence rate and a rebound in the QoL score. Therefore, the hybrid procedure may represent a better therapeutic option for VV patients [28]. In this study, the results suggest that both EVLA and RFA could relieve symptoms and improve QoL. Moreover, the VCSS and CIVIQ-20 scores exhibited greater improvements at 6 and 12 months after the hybrid EVLA procedure than after the RFA procedure, and the patients indicated greater satisfaction. These results suggest that the hybrid EVLA procedure could reduce recurrence and significantly improve patients’ QoL. Thus, we believe this real-world study contributes to an objective and realistic assessment of the clinical efficacy and outcomes of EVLA and RFA in treating VVs.
Our study has several limitations. First, this is not a randomized controlled study, and the conclusion might be influenced by the low quality of evidence-based medicine reports. Second, the sample size is small, and the results may be biased. Third, the follow-up period of this study is short; thus, the long-term outcomes of this procedure still need to be verified in further large-sample, multicentre, prospective, randomized, controlled studies. The differences among different thermal ablation procedures in the treatment of VVs still need to be evaluated by real-world studies involving more centres and larger samples, especially for cases of VVs without the GSV as the trunk. Thermal ablation procedures may be a hot research topic in the future, as it is very important to improve the clinical outcomes of thermal ablation procedures and reduce associated complications.
In conclusion, our findings suggest that both EVLA and RFA are effective therapies for VVs. However, the hybrid EVLA procedure can significantly reduce the recurrence of VVs in the calf and improve both QoL and patient satisfaction.