Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous lung condition characterised by chronic respiratory symptoms caused by abnormalities of the airways and/or alveoli that cause persistent, often progressive, airflow obstruction [1]. Due to variations in geographical location and survey analysis methods, the prevalence of COPD may exhibit differences. According to a systematic review and meta-analysis covering 8 countries in South Asia, the estimated prevalence of COPD is approximately 11.1%, with the highest prevalence in north India (19.4%) and Bangladesh (13.5%) [2]. Based on extensive epidemiological studies, the global incidence of COPD is estimated to be 10.3%, and the prevalence of COPD continues to increase [3, 4]. The development of COPD may result from the interplay between genetic predisposition and environmental factors [5]. The most significant genetic risk factor for COPD is mutations in the SERPINA1 gene, which leads to congenital α1-antitrypsin deficiency (AATD) [6]. Smoking, exposure to biomass, occupational dust, air pollution, body infections, and advancing age all represent significant risk factors for the development of COPD [7–12]. The World Health Organisation reported that COPD was the third leading cause of death globally in 2019 [13].
Current guidelines recommend long-acting β-agonists (LABAs), long-acting muscarinic receptor antagonists (LAMAs), inhaled corticosteroids (ICSs), and their combinations for patients with stable COPD to alleviate symptoms and improve quality of life [14]. Furthermore, various non-pharmacological treatments such as pulmonary rehabilitation are also available [15, 16]. Although these inhaled drugs can effectively reduce the risk of acute exacerbation in stable COPD patients, many individuals with triple therapy (ICS/LABA/LAMA) still experience repeated COPD episodes. A relatively high proportion of these patients have type 2 inflammation, which is characterised by elevated cytokines, including interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13) [17–23]. Type 2 inflammation contributes to the damage of the airway barrier, airway hyperresponsiveness, and airway remodeling in COPD patients [24–28]. Two previous randomised clinical trials (RCTs) showed that blocking IL-5 may not further improve the quality of life in patients with COPD using triple therapy [29, 30]. A recent study investigated that dupilumab, an IL-4 and IL-13 blocker, significantly reduced the risk of acute exacerbation in patients with COPD who were not well controlled on triple inhaler therapy [31]. However, the comparative efficacy of dupilumab, 3 conventional inhaled drugs, and their combinations in COPD patients remains incompletely undetermined. Due to practical factors, many head-to-head RCTs that directly compare the efficacy of 2 different drugs are difficult to perform. Network meta-analysis is an excellent method for indirectly comparing the efficacy of different drugs that lack head-to-head RCTs [32].
This study aims to employ network meta-analysis to compare the long-term efficacy of dupilumab with 3 conventional inhaled drugs and their combinations in mitigating acute exacerbation and all-cause mortality among stable COPD patients.
Material and methods
Search strategies
The study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines and extension statement of network meta-analysis [33]. A Literature search was performed in PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) databases (last search date: 14 June 2023). The complete search strategies are presented in Supplementary Table SI.
Selection criteria
The inclusion criteria of the study were as follows: (1) RCTs on ICS, LABA, LAMA, dupilumab, or their combinations; (2) adult patients with stable COPD; (3) the follow-up duration ≥ 48 weeks; and (4) published outcome information on death or exacerbation.
The RCTs were excluded if all the patients in the trial exhibited any of the following features: (1) same gender; or (2) comorbidity, such as cardiovascular diseases. The RCTs including partial patients with these characteristics were not excluded.
Study selection, data extraction, and bias assessment
Two researchers independently conducted study selection, data extraction, and bias assessment. Any differences were resolved through discussion. Literature management software was utilised to eliminate duplicate publications, and then ineligible literature was removed via the manual screening of titles and abstracts. Finally, the eligible studies were identified via the screening of full text. Information on the included studies was extracted, mainly including study design, population characteristics, interventions, and outcome measures. The potential for bias in each RCT was assessed using the Cochrane Risk of Bias Assessment Tool in 7 domains [34].
Outcome measures
The endpoints of this study included the number of patients with ≥ 1 acute exacerbation and the number of all-cause deaths during the study period. The 2 endpoints were effectiveness or safety endpoints in the included studies.
Statistical analysis
Bayesian network meta-analysis was used to amalgamate the direct and indirect evidence from the included studies and evaluate the comparative efficacy of multiple interventions [35]. Due to the potential heterogeneity among the abundant included studies, we used a random-effect model to estimate the posterior distributions that were presented as the odds ratios (ORs) and corresponding 95% confidence intervals (CIs). The I2 statistic was calculated to assess heterogeneity, with I2 > 50% as high heterogeneity. If a closed loop existed in the network graph, the node splitting method was used to determine inconsistency. A p-value of ≥ 0.05 indicated that the direct evidence in the network meta-analysis was consistent with the indirect evidence [36].
Several sensitivity analyses were used to explore the impact of studies with background medication interference, small sample size, or a relatively high risk of bias on the primary results. First, we reanalysed the comparative efficacy of different interventions after the removal of studies with background drug interference (defined as studies in which the drug of interest is included in the background treatment). Second, if more than 50% of patients in the trial had a mediation of drug classes of interest other than the study drug classes, the intervention type was classified as combination therapy (primary drug classes + background drug classes). Studies that indicated background drug interference during the study period but did not list the percentage of patients using background drugs at baseline were removed from the analysis. Third, network meta-analyses were performed after excluding relatively high-risk bias studies. Fourth, we reanalysed the study data after removing studies with sample sizes < 300 people.
All analyses were conducted using the “gemtc” package in R version 4.2.3 [36]. A two-sided p-value < 0.05 was considered statistically significant.
Results
Study selection and characteristics
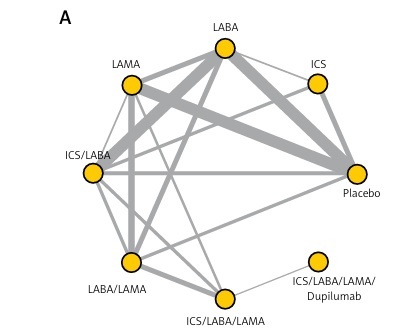
In this network meta-analysis, 8963 literature items were obtained by searching the database, and 69 RCTs, enrolling 125,331 COPD patients, were identified after stepwise screening (Supplementary Figure S1). 63 and 66 RCTs reported information on acute exacerbation and all-cause mortality, respectively, and the network plots of direct evidence are shown in Figure 1. Most of the studies were double-blind, placebo-controlled, multi-centre trials with a median duration of 12–48 months.
Figure 1
Network plots of direct evidence in the primary analysis: acute exacerbation (A) and all-cause mortality (B). Each node represents an intervention, and the connecting lines between the nodes represent the presence of direct comparisons; the thicker the line, the more studies are directly compared
LABA – long-acting β-agonist, LAMA – long-acting muscarinic receptor antagonist, ICS – inhaled corticosteroid.
The baseline characteristics were stratified based on the intervention type and presented in Figure 2. The distributions of age, gender, body mass index (BMI), and post-bronchodilation forced expiratory volume in 1 s as a percentage of the predicted value (post-FEV1%pred) were similar across the 8 different intervention types, demonstrating the similarity among diverse included studies. More detailed information about the studies are shown in Supplementary Table SII. Supplementary Figure S2 showed no remarkable bias risk in most studies.
Figure 2
The baseline characteristics. Baseline characteristics were stratified based on the 8 different intervention types, and the distributions are roughly the same
LABA – long-acting β-agonist, LAMA – long-acting muscarinic receptor antagonist, ICS – inhaled corticosteroid, BMI – body mass index, post FEV1%pred – post-bronchodilation forced expiratory volume in 1 s as a percentage of the predicted value.
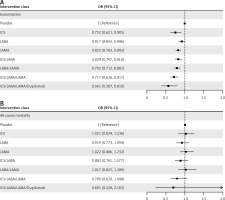
Primary analysis results
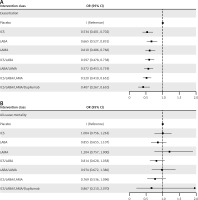
The primary analysis results are illustrated in Figure 3, and no high heterogeneity (I2 < 50%) or significant inconsistency (p > 0.05) was observed. As shown in Figure 3 A, seven drug therapies were significantly associated with a reduced risk of acute exacerbation in COPD patients compared with a placebo, and the reduction degree increased with the upgraded use of drug classes. ICS/LABA/LAMA/dupilumab was the most effective in reducing the risk of acute exacerbation (OR = 0.561 [95% CI: 0.387–0.810]), followed by the triple ICS/LABA/LAMA inhalation therapy (OR = 0.717 [95% CI: 0.626–0.817]). Seven drug treatments failed to significantly reduce all-cause mortality in COPD patients compared to placebo (Figure 3 B). Nonetheless, the estimate of ICS/LABA/LAMA/dupilumab compared with placebo was the smallest, denoting that ICS/LABA/LAMA/dupilumab may be optimal in all-cause mortality. More primary results are shown in Supplementary Table SIII.
Figure 3
The efficacies of interventions vs. placebo for 2 outcomes in the primary network meta-analysis, exacerbation (A), and all-cause mortality (B). The odds ratio (OR) and 95% credible interval (95% CI) are presented. OR < 1 favors intervention, and 95% CI not including 1 signifies statistical significance
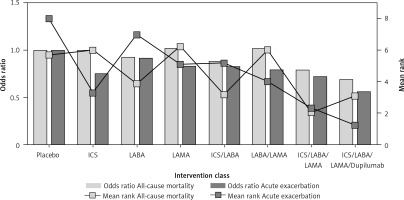
Ranking probabilities for each intervention on the 2 outcomes in the primary network meta-analysis are presented in Figure 4, and the ranking results in the 2 outcomes were consistent. ICS/LABA/LAMA/dupilumab was the most likely to be the optimal treatment, followed by ICS/LABA/LAMA, which is in line with the results in Figure 3. Furthermore, Figure 5 presents the odds ratio and mean ranks of all treatments for acute exacerbation and mortality. This illustrates that using combinations of medications gradually leads to improved long-term outcomes in acute exacerbation but does not affect mortality in patients with COPD.
Figure 4
Ranking probabilities for each intervention on the 2 outcomes in the primary network meta-analysis, acute exacerbation (A), and all-cause mortality (B)
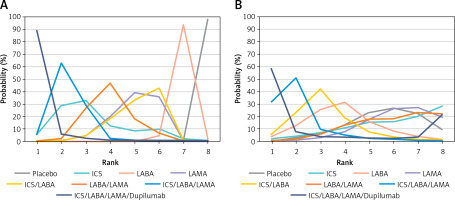
Figure 5
The odds ratio and mean ranks of all interventions for acute exacerbation and all-cause mortality. The point estimates (odds ratio) of interventions compared with placebo for acute exacerbation and all-cause mortality are presented with bars. The mean ranks estimated from the ranking probabilities are summarised by lines. The more effective intervention has a lower mean rank
Sensitivity analysis results
Four sensitivity analysis results are presented in Figure 6, Supplementary Table SIV, and Supplementary Figure S3, and no significant heterogeneity or inconsistency was observed. The results of multiple sensitivity analyses consistently showed that ICS/LABA/LAMA/dupilumab was the most effective in reducing the risk of acute exacerbation, in line with those of primary analyses.
Figure 6
The results of four sensitivity analyses. First, the removal of studies with background drug interference (A, B). Exacerbation (A, C, E, G) and all-cause mortality (B, D, F, H). The odds ratio (OR) and 95% credible interval (95% CI) are presented. OR < 1 favors intervention, and 95% CI not including 1 signifies statistical significance, Second, dealing with background drugs (C, D). Third, excluding relatively high risk of bias studies (E). Exacerbation (A, C, E, G) and all-cause mortality (B, D, F, H). The odds ratio (OR) and 95% credible interval (95% CI) are presented. OR < 1 favors intervention, and 95% CI not including 1 signifies statistical significance, Third, excluding relatively high risk of bias studies (F). Fourth, removing studies with sample sizes < 300 (G, H). Exacerbation (A, C, E, G) and all-cause mortality (B, D, F, H). The odds ratio (OR) and 95% credible interval (95% CI) are presented. OR < 1 favors intervention, and 95% CI not including 1 signifies statistical significance


Discussion
This study is the first network meta-analysis to compare the efficacy of therapy containing dupilumab with conventional inhaled therapy in the long-term risk of acute exacerbation and all-cause mortality in COPD patients. Primary analyses have shown that 7 drug therapies were significantly related to a reduction in the risk of exacerbation but not death in COPD patients. ICS/LABA/LAMA/dupilumab was the optimal drug therapy for reducing the risk of acute exacerbation in patients with COPD. The results of sensitivity analyses did not contradict those of primary analyses.
A previous network meta-analysis of RCT on ICS, LABA, and LAMA found that ICS/LABA/LAMA was significantly associated with lower risks of total exacerbations and all-cause mortality in stable COPD patients compared with placebo, and this was the optimal treatment among the conventional therapies [37]. However, single therapies of ICS, LABA, and LAMA showed no significant decrease in all-cause mortality. The contradiction between triple therapy and single therapies makes clinicians quite confused about the drug components through which triple therapy reduces the risk of all-cause mortality. Despite the statistical significance of triple therapy in reducing all-cause mortality, this result is likely to be biased. The previous meta-analyses simultaneously included short-term and long-term studies, and over 60% of the RCTs included in the study had a follow-up of < 1 year. A proportion of included studies enrolled the whole population with high cardiovascular diseases or other severe comorbidities, affecting the similarity of the population. Background medication interference was not considered in that analysis. These potential biases probably contributed to the contradicted results. Our study found different results from the previous study after decreasing these potential biases, i.e. that all conventional inhaled therapies were insignificantly associated with a decrease in all-cause death. Furthermore, the combination of triple conventional therapy with dupilumab cannot also significantly reduce all-cause death, further denoting that the current drug therapies were ineffective in decreasing long-term mortality.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023 guideline states that triple-inhaled therapy is more effective than single or dual inhaled therapies in the prevention of acute exacerbation [14]. The results of our study are consistent with the guideline recommendation. Two previous RCTs, IMPACT and ETHOS, showed a significant reduction in all-cause mortality with ICS/LABA/LAMA compared to LABA/LAMA, and thus ICS/LABA/LAMA is recommended to decrease the death risk of COPD patients. However, no direct evidence showed that ICS/LABA/LAMA or LABA/LAMA was superior to placebo in all-cause mortality. Our study suggests that the association of ICS/LABA/LAMA with reduced long-term mortality risk may not be significant.
Although the current triple inhaled therapy can effectively prevent COPD exacerbation, many patients with standard triple therapy still experience recurrent acute exacerbations due to type 2 inflammation. IL-4, IL-5, and IL-13 were deemed as key anti-inflammation targets. Recent RCTs revealed that blocking IL-4 and IL-13 (dupilumab) but not IL-5 (benralizumab or mepolizumab) can significantly reduce the acute exacerbation risks in COPD [29–31]. Dupilumab dually blocks both IL-4 and IL-13 to block the activation of antigen-presenting cells induced by Th2 cells, along with the expression of eosinophils, inflammatory cytokines, and chemokines in vivo in the lungs. Dupilumab can block type 2 inflammation in a broader range compared to IL-5 blockers, which is expected to result in more significant benefits in reducing COPD exacerbations [38, 39]. This study pooled nearly 20 years of RCTs to indicate that ICS/LABA/LAMA/dupilumab is expected to be the most effective medication for reducing the risk of acute exacerbation in patients with stable COPD.
The therapy of COPD patients is determined based on the COPD severity [14]. As the severity of COPD increases, the number of classes of drugs used also increases. Our study demonstrated that an increase in the type of medication used contributes to gradual benefit in acute exacerbation but not death in COPD, and ICS/LABA/LAMA/dupilumab was the optimal therapy. For patients with poorly managed conditions under the standard triple inhaled medication and type 2 inflammation, ICS/LABA/LAMA/dupilumab should be recommended.
This study has some limitations. First, despite our thorough evaluation of bias, the internal selection bias of the RCT could not be entirely eradicated. Therefore, the results of this study may not apply to all COPD patients in a clinical context. Second, our analyses did not take the specific drugs and dosages into account, which may have an unknown impact on our results. Additionally, we did not include acute moderate-to-severe exacerbations in the outcome analyses owing to the lack of data, and thus the estimation of efficacy is slightly incomplete. Finally, only one eligible RCT on dupilumab was identified and included in this meta-analysis, probably affecting the evidence power.
In conclusion, gradually increasing the number of these drug combinations led to a significant decrease in the long-term risk of acute exacerbation in individuals with COPD. However, mortality rates remained unaffected. The ICS/LABA/LAMA/dupilumab combination was the most effective in COPD patients.