Introduction
Acute pancreatitis (AP) is one of the most common and critical diseases of the digestive system. It has been reported that 5–35 people per 100,000 seek medical attention for AP each year, and the incidence has been on the rise for the past two decades [1, 2]. Approximately 15–20% of patients with AP develop moderate or severe disease. At this level of severity, patients may develop multi-organ failure, with a mortality rate of 20–40%, and these patients often require admission to an intensive care unit (ICU) [3]. Meanwhile, AP is exceptionally prone to recurrence when the underlying cause of its onset is not found or when the underlying cause is not eliminated, and its recurrence rate is between 10% and 30% [4, 5]. Recurrent acute pancreatitis (RAP) affects patients’ quality of life and increases the burden of healthcare costs for patients [6]. In addition, RAP is also a significant risk factor for progression to chronic pancreatitis [7]. Other studies have suggested that RAP may be associated with pancreatic cancer [8].
A study by Lee et al. [4] retrospectively analyzed the clinical outcomes of 292 patients with AP (213 patients with IAP and 79 patients with RAP) who attended the Cleveland Clinic between 2008 and 2011 and found a mortality rate of 4.7% for IAP patients and 0% for patients with RAP (p = 0.047). The investigators concluded that patients with RAP may be at reduced risk of a clinically severe course and have reduced mortality. In addition, after adjusting for potential confounders (e.g., transfer status, obesity), they found that prior episodes of AP were protective against multisystem organ failure and admission to the ICU in RAP. However, few studies have compared the differences between RAP and IAP, and there is a lack of data related to RAP admission to the ICU. This study is intended to further elucidate the differences between IAP and RAP based on a large public database (the Medical Information Mart for Intensive Care, MIMIC-IV), to provide clinical evidence on allocating healthcare resources related to AP.
Material and methods
Introduction to the database
The MIMIC is a database of intensive care medicine, established in 2003 with funding from the National Institutes of Health (NIH) by emergency physicians, intensivists, and computer science experts from Beth Israel Deaconess Medical Center, Massachusetts Institute of Technology, Oxford University, and Massachusetts General Hospital [9], and has been updated to version 4 (MIMIC-IV, https://mimic.mit.edu/). The MIMIC-IV database currently collects information on more than 70,000 critical care hospitalizations, which is far more cases than any single-center clinical trial site worldwide. The data collection and entry process of the MIMIC-IV is done by professionally trained personnel and can be considered a high-quality multi-center clinical research database.
Study population
Patients with AP were identified according to the ICD codes of the diagnosis and those admitted to the hospital or ICU for chronic pancreatitis were excluded from this study. The following information was extracted for included patients using Navicat software (version 16.1.3): age, gender, race (white, black, and other ethnicities), Charlson Comorbidity Index, presence of acute kidney injury/sepsis/obesity, BISAP (Bedside Index for Severity in Acute Pancreatitis) score, SIRS (Systemic Inflammatory Response Syndrome) score, LODS (Logistic Organ Dysfunction System) score [10], OASIS (Oxford Acute Severity of Illness Score) score [11], SAPS II (Simplified Acute Physiology Score II) score [12], laboratory tests (hemoglobin, red blood cells, red blood cell distribution width, platelets, white blood cells, anion gap, blood urea nitrogen, creatinine, international normalized ratio, prothrombin time, alanine aminotransferase, aspartate aminotransferase, total bilirubin, and blood glucose), vital signs (heart rate, mean arterial pressure, respiratory rate, and body temperature), fluid intake and urine output on the first day of admission. The BISAP score [13] was introduced in 2008 and is cumulative with the presence of the following: blood urea nitrogen > 25 mg/dl, impaired mental status (Glasgow Coma Score < 15), SIRS, age > 60 years, and presence of pleural effusion. The BISAP score has been shown to be useful for the early identification of AP with an increased risk of in-hospital death [14–16]. However, the relationship between BISAP score and prognosis of severe AP lacks large-scale data support.
RAP was defined as acute pancreatitis that occurred at least 2 months after the last episode [4, 17, 18]. The time difference between the patient’s admissions was calculated using Python software (version 3.9), and the diagnosis of RAP was rejected if the time difference between the two hospitalizations was less than 2 months.
Statistical analysis
Statistical analyses were performed using R software (version 4.1.2) and MedCalc software (version 20.1.0). Patients with IAP and RAP were grouped, and their basic characteristics were described. It was first determined whether continuous variables conformed to a normal distribution using the Kolmogorov-Smirnov test. If they conformed to a normal distribution (presented as mean ± standard deviation), Student’s t-test was performed for comparison between groups, and if they did not conform to a normal distribution (presented as median and interquartile range), a non-parametric test (Mann-Whitney U test) was performed for comparison between groups. Categorical variables (presented as sample size and percentages) were compared between groups using the χ2 test. Kaplan-Meier curves were plotted to determine whether there was a difference in survival between the two groups by the log-rank test and Tarone-Ware test. Binomial logistic regression analysis was performed to identify independent risk factors for in-hospital mortality of the patients, where variables with p-values < 0.1 in the univariable regression analysis were included in the multivariable regression analysis. The predictive value of the four scoring systems (LODS, OASIS, and SAPS II have all been used for prognostic prediction in patients admitted to the ICU) for in-hospital mortality of the patients was further compared by plotting the receiver operating characteristic (ROC) curves of each scoring system, and the area under the curves (AUCs) were tested for differences by the method of Delong et al. The decision curve analysis (DCA) was also performed to determine the net clinical benefit of each scoring system when applied to AP patients. P-values less than 0.05 were considered statistically different.
Results
Epidemiological features of RAP
We identified 6195 patient admissions with a diagnosis of AP from over 200,000 admissions in the MIMIC-IV database between 2008 and 2019. After excluding repeat hospitalizations, a total of 4060 patients were diagnosed with AP, 541 of whom were readmitted for RAP, and the time interval between the second episode of AP and the initial episode was 154 (90–443) days. There were 151 in-hospital deaths (in-hospital mortality rate of 4.29%) in patients with IAP and 4 in-hospital deaths (in-hospital mortality rate of 0.74%) in patients with RAP, with a statistically significant difference (p < 0.001) and an overall in-hospital mortality rate of 3.82%. There were 1344 admissions to the ICU (over 70,000 ICU admissions in the database) with AP, and after excluding repeat admissions, there were 1030 independent individual patients with the specific diagnoses shown in Table I. Of these 1030 ICU admissions, 974 patients were diagnosed with IAP, of whom 79 were diagnosed with biliary AP, 63 with alcohol-induced AP, 6 with drug-induced AP, and 5 with idiopathic AP; the other 56 patients were diagnosed with RAP, of whom 5 were diagnosed with alcohol induced AP, 1 with biliary AP, and 1 with drug-induced AP, and 5 with idiopathic AP, while the etiology of the remaining 49 patients was unknown.
Table I
Diagnosis of included patients
Baseline characteristics of included patients
Patients with RAP were younger, had a lower Charlson Comorbidity Index, lower BISAP and SIRS scores, and lower hemoglobin, blood urea nitrogen, creatinine, alanine aminotransferase, aspartate aminotransferase, and total bilirubin levels than those with IAP. The remaining baseline characteristics were not significantly different between the two groups (Table II).
Table II
Baseline characteristics of patients
[i] IAP – initial acute pancreatitis, RAP – recurrent acute pancreatitis, CCI – Charlson comorbidity index, AKI – acute kidney injury, BISAP – Bedside Index for Severity in Acute Pancreatitis, SIRS – systemic inflammatory response syndrome, RBC – red blood cells, RDW – red cell distribution width, WBC – white blood cells, BUN – blood urea nitrogen, INR – international normalized ratio, PT – prothrombin time, ALT – alanine aminotransferase, AST – aspartate aminotransferase, TBil – total bilirubin, MAP – mean artery pressure, UO – urine output.
Outcomes of included patients
Among the included patients admitted to the ICU, the in-hospital mortality rate was 13.96% (of the 974 patients, 136 died) for patients with IAP and 3.57% (of the 56 patients, 2 died) for RAP. The risk of in-hospital death was lower for RAP (RR = 0.892, 95% CI: 0.843–0.944), and the difference was statistically significant (p = 0.025). Patients in the IAP group were hospitalized for 10.7 days (5.8–20.0 days) and stayed in the ICU for 2.6 days (1.2–6.0 days); patients in the RAP group were hospitalized for 8.8 days (5.8–18.3 days) and stayed in the ICU for 2.3 days (1.3–4.5 days). There was no significant difference between the two groups regarding length of hospital stay and length of stay in the ICU (Table III).
Table III
Outcomes of patients
| Outcomes | IAP (n = 974) | RAP (n = 56) | Relative risk* (95% CI) | P-value |
|---|---|---|---|---|
| Death in hospital | 136 (13.96) | 2 (3.57) | 0.892 (0.843–0.944) | 0.025 |
| LOS hospital (day) | 10.7 (5.8–20.0) | 8.8 (5.8–18.3) | / | 0.507 |
| LOS ICU (day) | 2.6 (1.2–6.0) | 2.3 (1.3–4.5) | / | 0.497 |
Survival analysis and independent risk factors for in-hospital mortality
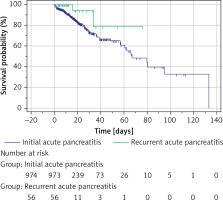
Kaplan-Meier curves were plotted for the survival of the two groups (Figure 1). P = 0.064 for the log-rank test and p = 0.048 for the Tarone-Ware test. Since the log-rank test is more sensitive to differences in distant outcome events, the difference in survival between the two groups is considered statistically significant here. The median survival time was 66.9 days (60.8–133.2 days) for patients with IAP and could not be calculated for patients with RAP (due to too few deaths), whose mean survival time was 66.214 days (standard deviation of 6.874 days).
Figure 1
Kaplan-Meier curves of patients with initial and recurrent acute pancreatitis in the intensive care unit
The results of the binomial logistic regression analysis showed that for IAP, the Charlson Comorbidity Index and the BISAP/SIRS score on the first day may be independent risk factors for in-hospital mortality (Table IV). Here, we tested for covariance between age, BISAP, and SIRS using a linear regression equation with variance inflation factor (VIF) values of 1.544, 1.648, and 1.155, respectively, confirming the absence of covariance. However, age, sex, Charlson Comorbidity Index, BISAP/SIRS score on the first day, and the presence of obesity were not independent risk factors for in-hospital mortality in patients with RAP (Table V). We also observed that RAP was not an independent risk factor for in-hospital mortality relative to IAP after adjusting for a range of confounders (Table VI).
Table IV
Binomial logistic regression analysis for in-hospital mortality among patients with initial acute pancreatitis
Table V
Binomial logistic regression analysis for in-hospital mortality among patients with recurrent acute pancreatitis
Table VI
Binomial logistic regression analysis for in-hospital mortality among intensive care patients with acute pancreatitis
Scoring system selection for predicting in-hospital mortality
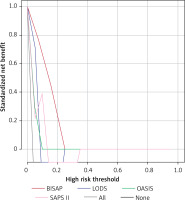
For patients with IAP, the ROC results of the four scoring systems are shown in Figure 2. The AUC values, optimal cutoff values, sensitivity, specificity, and Youden index of the four scoring systems are presented in Table VII, with the following Z-test results: BISAP vs. LODS with a Z value of 5.950, p < 0.0001; BISAP vs. OASIS with a Z-value of 3.785, p = 0.0002; BISAP vs. SAPS II with a Z-value of 5.838, p < 0.0001; LODS vs. OASIS with a Z-value of 2.647, p = 0.0081; LODS vs. SAPS II with a Z-value of 0.183, p = 0.8545; OASIS vs. SAPS II with a Z-value of 2.710, p = 0.0067. In the DCA curves (Figure 3), the net clinical benefit of SAPS II was almost always higher than that of the other scoring systems in the threshold range of 0.2–0.6. However, none of the four scoring systems showed a net clinical benefit above the threshold of 0.6.
Table VII
Comparison of ROC curves (initial acute pancreatitis)
Figure 2
ROC curves of four scoring systems for predicting in-hospital mortality in initial acute pancreatitis
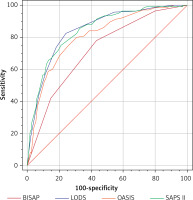
Figure 3
DCA curves of four scoring systems for predicting in-hospital mortality in initial acute pancreatitis
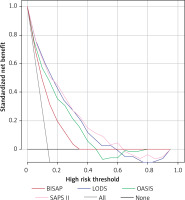
For patients with RAP, the ROC results of the four scoring systems are shown in Figure 4 and Table VIII, with Z-test results of BISAP vs. LODS with a Z-value of 2.427, p = 0.0152; BISAP vs. OASIS with a Z-value of 1.418, p = 0.1562; BISAP vs. SAPS II with a Z-value of 0.843, p = 0.3991; LODS vs. OASIS with a Z-value of 0.976, p = 0.3288; LODS vs. SAPS II with a Z-value of 0.234, p = 0.8149. OASIS vs. SAPS II, Z-value 2.497, p = 0.0125. In the DCA curves (Figure 5), the net clinical benefit of BISAP was almost always higher than that of the other scoring systems in the threshold range of 0-0.25. However, none of the four scoring systems showed a net clinical benefit in the other threshold ranges.
Table VIII
Comparison of ROC curves (recurrent acute pancreatitis)
Discussion
This study is one of the first to investigate the prognostic differences in patients with AP in the ICU. Our results were similar to those of Lee et al. [4], in that patients with RAP had lower severity (lower BISAP and SIRS scores on the first day of admission) and a lower risk of in-hospital death than those with IAP. In addition, consistently with previous studies, patients with RAP were younger and had a lower Charlson Comorbidity Index. This index [19] can be used to assess the impact of co-morbidities other than the underlying disease that is currently the primary focus of treatment for the future survival of patients. It seems that we could attribute the lower mortality in patients with RAP to lower age and a lower value of the Charlson Comorbidity Index. However, the results of binomial logistic regression analysis suggest that age and the Charlson Comorbidity Index are not independent risk factors for in-hospital mortality in patients with RAP, and only the Charlson Comorbidity Index is independently associated with in-hospital mortality in patients with IAP. The answer to the question of why patients with RAP are more likely to be younger and have a lower comorbidity index is not yet available from previous studies. It needs to be further explored at a later stage. In addition to blood urea nitrogen, we also found that patients with RAP had lower creatinine levels, alanine aminotransferase levels, aspartate aminotransferase levels, and total bilirubin levels, which to some extent reflect the liver and kidney function of the patients, suggesting that better liver and kidney function in patients with RAP may also contribute to the low mortality rate. However, from the causal inference perspective, we cannot yet explain why patients with RAP have better hepatic and renal function.
Understanding the differences between IAP and RAP at the pathogenesis level can help provide better treatment options for patients. The reason for the lower severity of disease in patients with RAP may stem from the loss of alveolar cells and pancreatic fibrosis due to each episode of pancreatitis. As a direct result of reduced alveolar cells, there may be less pancreatic auto-digestion and necrosis, and subsequently a lesser inflammatory cascade response [20]. In contrast, pancreatic fibrosis has been shown to directly reduce the severity of acute-on-chronic pancreatitis [21]. Other researchers suggested that the protective immune mechanism of the body is not activated during IAP, and this protective immune mechanism may protect the body in RAP [4]. However, starting from the three possible mechanisms mentioned above, only enhancing protective immune mechanisms is a potential therapy. With the flourishing development of molecular biology technologies, including genomics, proteomics, and transcriptomics, in recent years, there is reason to believe that the essential differences between IAP and RAP (e.g., details on differentially expressed genes, protein expression levels, and key transcription factors in the development of the disease course in both types of AP) will be further elucidated, thus providing robust evidence for precision medicine in AP.
For prognostic prediction of patients with AP admitted to the ICU, Huang et al. developed a nomogram that showed good predictive performance [22]. We investigated the predictive value of four preexisting scoring systems in the prognosis of patients with AP, in which LODS, OASIS, and SAPS II were all used as prognostic predictive scoring systems in the ICU and also showed good predictive value [23]. For patients with IAP, SAPS II appears to be the superior predictive scoring system, and although it has the highest AUC (0.847) and the highest Youden index (0.5825), it is equivalent in value to LODS in the Z-test. However, the DCA curve suggests that the net clinical benefit for patients may be higher when using the SAPS II score as a predictive scoring system. DCA curves have been used extensively to evaluate the clinical utility of a model, i.e., whether the model is worthy of being practiced clinically [24–27]. The value of each DCA curve can be described using the net benefit ratio, the magnitude of which is similar to the AUC of the ROC curve, i.e., the larger the area under the DCA curve, the larger the net benefit ratio. As seen from Figure 3, if we choose the threshold probability range of 0.2–0.6 corresponding to the horizontal coordinate, SAPS II almost always outperforms the other three scoring systems. In the range greater than 0.6, all scoring systems have no significant net benefit. However, for RAP, the results were quite different, and BISAP appeared to show some advantage in predicting the prognosis of RAP. The AUC value (0.944) and the Youden index (0.8889) were the highest when using the BISAP score for prognosis prediction of RAP. However, compared to the other three scoring systems, there was only a significant difference with the AUC of LODS. Afterward, the DCA curve results showed that the net benefit of BISAP was most significant between the threshold probabilities 0 and 0.25. In conclusion, the prognostic prediction should not be generalized for patients with AP admitted to the ICU. In the case of patients with IAP, selecting a critical care scoring system for prognostic prediction may be a better choice, while in patients with RAP, the BISAP score may have some advantages. Approximately 14–20% of patients with AP are reported to require intensive care due to multi-organ dysfunction and/or failure, and multidisciplinary teamwork in intensive care can reduce mortality from 30% to 10% in severe AP [28]. It is valuable to clarify the clinical features of AP in intensive care, and considering that patients with first-episode AP may be more severely ill, we believe that the importance of intensive care in the management of patients with first-episode AP should be emphasized to prevent the deterioration of the patient’s condition in advance. In fact, there is no sufficiently reliable prognostic score to predict the occurrence of severe AP. The Guidelines for the Management of Patients with Severe Acute Pancreatitis, 2021 state that the BISAP score is likely the most appropriate predictor of the development of severe AP [29]. We demonstrated the potential of the BISAP score in the prognosis prediction of RAP, further enriching the clinical application value of BISAP. However, only a few previous studies related to intensive care in RAP have been reported, and our study also fills this gap to some extent.
Even though both this study and the study by Lee et al. [4] suggest that patients with RAP may have a milder disease than the initial attack, their relatively high mortality rate is still unacceptable to us. Determining the etiology of an acute pancreatitis attack is a key factor in preventing recurrence. Among the 56 patients with RAP included in this study, as we have previously stated, 5 cases were definite alcoholic AP (about 9%), while there was 1 case each of drug-induced AP and biliary AP, and the etiology of the remaining patients was unclear. It has been observed that after the first episode of alcoholic AP, 46% of patients experience at least one recurrence during 10–20 years of follow-up, along with an increased risk of developing chronic pancreatitis [30, 31]. In addition, personal alcohol consumption is not associated with RAP, nor is the type of alcoholic beverage associated with RAP [31]. However, there is definite evidence that smoking and obesity are risk factors for alcohol-induced RAP [32, 33]. Therefore, in patients with alcoholic AP, weight control and smoking cessation may be effective measures to prevent a recurrence. For biliary AP, removing the gallbladder is necessary to prevent recurrence [34]. As for drug-induced AP, discontinuation of potentially pathogenic drugs and follow-up may be helpful for patients. Some drugs have also been used for the prevention of RAP, including octreotide, pancreatic enzymes, and ursodeoxycholic acid [34], but there is a lack of high-quality, evidence-based medical evidence. However, most opinions believe that the etiology of idiopathic AP is biliary microstones or sphincter of Oddi dysfunction, which cannot be detected by conventional methods [34], and laparoscopic cholecystectomy and necessary genetic testing may help to reduce recurrence [35]. In addition to the causes mentioned above, anatomical variants of the pancreas and genetic mutations are also possible causes of the development of AP [36]. Another study showed that AP is prone to recurrence even during treatment, and factors such as uncontrolled systemic inflammatory response may be responsible for recurrence in such patients [6]. It must be emphasized that AP recurrence is likely to result from a combination of factors [6] and any cause of AP that is not adequately corrected may lead to recurrent attacks. In a word, to reduce the occurrence of RAP, primary, secondary, and tertiary preventive measures should be systematically implemented to mitigate the effects of AP and its sequelae as soon as possible. Personal education of patients, effective in-hospital management, and screening of high-risk patients all contribute to the prevention of RAP [37].
All in all, the strength of this study is that the study population was derived from a large clinical database, presenting the clinical characteristics of RAP in intensive care and the independent risk factors affecting their mortality in the largest possible sample size, as well as comparing the scoring systems related to the prediction of their mortality and comparing them more comprehensively with the characteristics of patients with IAP during the same period. The current study population for RAP originates only from general gastroenterology and is instructive for the management of AP patients in the ICU. Moreover, we report for the first time that there was no significant difference between patients with IAP and patients with RAP in terms of length of hospitalization and length of stay in the ICU as secondary outcomes. Meanwhile, we confirmed that for the prognostic prediction of RAP patients, the BISAP score possesses a greater advantage, and can achieve a greater net clinical benefit for patients while ensuring predictive efficacy. Therefore, the BISAP scoring system may be the preferred option for prognostic prediction of RAP patients in future clinical practice. However, we must acknowledge certain limitations of this study. Firstly, we did not explore the relationship between the number of episodes and the prognosis of AP, due to the extensive time span of this database and the lack of uniformity in follow-up, which makes it difficult to normalize the number of episodes. Secondly, this study is based on the US population and it remains unknown whether all the conclusions are applicable to populations in other countries or regions. Furthermore, due to the large number of missing values in the database for amylase, lipids (some studies have shown that elevated LDL cholesterol level is an independent risk factor for RAP [5]) and other laboratory tests, and the unavailability of imaging data (including whether the pancreas was necrotic, formed pseudocysts or abscesses, etc.) of the patients, the impact of these indicators on the outcomes was not explored, which may affect the stability of the results. Also, we were unable to grade the patients in terms of severity based on methods such as the Atlanta Classification. Unfortunately, as shown in Table I, the etiology of most patients was also unknown to us. Lastly, considering the small number of patients in the RAP group, the stability of the results remains to be tested. Therefore, a rigorously designed prospective randomized controlled clinical trial with a large sample is still essential to thoroughly assess the differences between IAP and RAP.
In conclusion, RAP was less severe and had a lower risk of in-hospital mortality than IAP. For IAP, the Charlson Comorbidity Index and the BISAP/SIRS score on the first day of admission were independent risk factors for in-hospital death; no independent risk factors for in-hospital death in patients with RAP were identified in this study. The SAPS II score is a better scoring system for predicting in-hospital mortality in patients with IAP. In contrast, the BISAP score showed some potential in predicting in-hospital mortality in patients with RAP.