Introduction
Chronic obstructive pulmonary disease (COPD) is a complex and heterogeneous syndrome that is often misdiagnosed, since there are many different coexisting mechanisms underlying its pathogenesis [1]. Nowadays, COPD diagnosis is confirmed by the presence of airflow limitation, while main contributors for disease development are tobacco smoking as well as biomass fuel exposure, air pollution and genetic factors [2]. Cigarette smoke causes oxidative stress and therefore contributes to the COPD inflammation and its progression [3]. Moreover, it was also reported that the level of oxidative stress remains high even in former smokers [4]. Although COPD inflammatory responses are localized to peripheral airways and lung parenchyma, systemic inflammation was observed in some patients as well, and it was associated with disease progression [5].
Many studies have investigated differences between COPD patients and healthy subjects in white blood cell (WBC) count and specific types of leukocytes, such as neutrophils (NEUTRO), lymphocytes (LY), monocytes (MO), eosinophils (EO) and basophils (BA). Moreover, it was suggested that ratios of blood cell types could minimize variations associated with measurement, sample handling and therapies, and yield more accurate diagnostic performances over blood cell counts [6, 7]. Therefore, neutrophil to lymphocyte ratio (NLR), derived NLR (dNLR), monocyte to lymphocyte ratio (MLR), basophil to lymphocyte ratio (BLR) and monocyte/granulocyte to lymphocyte ratio (M/GLR) are some of the ratios recognized as potential markers of inflammation. Neutrophil to lymphocyte ratio had already been investigated in many inflammatory diseases, predominantly in malignant diseases [8–13] and in compliance with smoking status [14]. It was also recognized as a useful marker for the assessment of FEV1-based COPD severity [15, 16]. Moreover, it was observed that symptoms and quality of life deteriorated with the increase of NLR [17]. Neutrophil to lymphocyte ratio had also successfully predicted the mortality of patients with COPD exacerbations [18–22]. However, only two groups of researchers evaluated an association between NLR and clinically useful modified Medical Research Council (mMRC) dyspnoea score or COPD multicomponent BODE index [23, 24]. On the other hand, derived NLR (dNLR) is a modification of NLR [9], and it had also been predominantly investigated in cancers and lymphoma [10, 25–27]. Only one paper reported about dNLR in COPD and showed its association with in-hospital mortality after exacerbations [28].
Besides dNLR, another modified NLR parameter is M/GLR [27], and potential roles of dNLR and M/GLR in stable COPD have not been investigated so far. Concerning other blood cell ratios, MLR was shown to be a good predictor in different malignant diseases [18, 29, 30] as well as a useful inflammatory marker for tuberculosis and autoimmune diseases [31], while there are no statistically significant data about its role in COPD [28]. Regarding the role of basophils, they might be involved in an increase of interleukin (IL)-4 that causes differentiation of monocytes to macrophages, which then lead to the destruction of alveolar walls and emphysema [32]. The relation of basophils to smoking and FEV1 in COPD patients was observed [33], yet there are no data about basophil-related ratios associated with smoking history and COPD.
Although there are many different studies investigating blood cells as potential biomarkers in a wide variety of diseases, many things still need to be clarified for the better treatment of COPD patients. Current Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) recommendations about COPD therapy include short-acting β2-agonists (SABAs) or short-acting muscarinic antagonists (SAMAs), long-acting β2-agonists (LABAs) and long-acting muscarinic antagonists (LAMAs) that improve lung function, or inhaled corticosteroids (ICS) that are often combined with long-acting bronchodilators. To the best of our knowledge, data regarding the impact of various bronchodilators alone or in combination with ICS on leukocyte subpopulations are lacking in COPD.
This study aimed to assess differences in WBC, NEUTRO, LY, MO and BA as well as in ratios of various leukocyte subtypes (NLR, dNLR, M/GLR, NMR, MLR, BNR, BLR and BMR) between stable COPD patients and healthy individuals, especially since there are data only about NLR in stable COPD. As no one had compared NLR, dNLR and M/GLR in COPD, we wanted to investigate whether there are any differences between them when concerning FEV1-based disease progression, symptoms severity and association with lung function, dyspnoea or the multicomponent COPD indices BODCAT and DOSE. Moreover, we wanted to evaluate the potential of leukocyte subsets and their various ratios in COPD prediction. Finally, we explored the effects of therapy regimes on leukocyte parameters, as this information is lacking for COPD.
Material and methods
Participants
There were 204 participants included in the cross-sectional, case-control study. One hundred and nine of them were COPD patients and 95 of them were age- and sex-matched healthy subjects. All participants agreed to volunteer and signed informed consent for the scientific research that they took part in.
The study was approved by the Ethical Committee of University Hospital Centre Zagreb (Zagreb, Croatia) and by the Ethical Committee for Experimentation of the Faculty of Pharmacy and Biochemistry, University of Zagreb (Zagreb, Croatia).
Participants were recruited during 2017 and 2018 at the Clinical Department for Lung Diseases Jordanovac, University Hospital Centre Zagreb according to the detailed inclusion and exclusion criteria. COPD diagnosis was confirmed by a pulmonology specialist based on anamnesis and clinical examination, current symptoms, and spirometry during the recruitment, while health status of the control group was established based on anamnesis and spirometry analysis. All COPD patients were in a stable phase of the disease with no exacerbations during the last 3 months, without any changes in therapy regime and without infections in the lower respiratory tract. GOLD recommendations [2] suggest that patients with FEV1/FVC < 0.70 should be subdivided into stages as follows: patients with FEV1 ≥ 80% as GOLD 1 (n = 0), with 50% ≤ FEV1 < 80% as GOLD 2 (n = 39), 30% ≤ FEV1 < 50% as GOLD 3 (n = 36), and FEV1 < 30% as GOLD 4 (n = 34). Moreover, COPD patients were grouped based on symptoms severity and exacerbation history in GOLD (GOLD A, n = 14; GOLD B, n = 63; GOLD C, n = 0; GOLD D, n = 32) groups based on the score of the COPD Assessment Test (CAT) that was used for the symptoms assessment. Participants from both groups had to be older than 40 years, could not have any lung diseases (except COPD for COPD patients), could not have any inflammatory diseases, manifest cardiovascular diseases, acute infections, diabetes with severe complications, severe liver diseases, severe kidney insufficiencies, malignant diseases, transplantations and other specific or non-specific ongoing inflammation. In addition, self-reported data about smoking status were collected from both groups. According to smoking history, controls were subdivided into healthy non-smokers (n = 48) and healthy smokers (n = 47), while COPD patients were subdivided into COPD non-smokers (n = 5), COPD former smokers (n = 75) and COPD smokers (n = 29). Chronic obstructive pulmonary disease patients who quit smoking at least 6 months before recruitment to the study were considered former smokers. The mMRC Dyspnoea Scale and CAT questionnaires were completed by COPD patients. Additionally, data about body mass index (BMI) and number of exacerbations in the previous year were collected. BODCAT and DOSE were calculated. BODCAT is a multicomponent COPD index that consists of BMI, airflow obstruction, dyspnoea and CAT score, while DOSE consists of dyspnoea, airflow obstruction, smoking status and number of exacerbations in the previous year [34].
Measurement of inflammatory markers
Samples from all participants were collected between 7 and 9 a.m. into the tubes by venepuncture of a large antecubital vein after overnight fasting, as recommended. The venepuncture procedure and order of blood sampling and handling were performed according to the national recommendations for venous blood sampling [35]. Complete blood count (CBC) was performed from the sample in the EDTA tube within 0.5 h from blood sampling. A sample in the tube with clot activator and gel separator for serum CRP determination was centrifuged at 2000 × g for 10 min, while a sample in the tube with sodium citrate for plasma Fbg determination was centrifuged twice at 1500 × g for 15 min, as recommended by national recommendations [36]. Afterwards, C-reactive protein (CRP) and fibrinogen (Fbg) were measured on a Cobas c501 analyser (Roche Diagnostics GmbH, Mannheim, Germany) and BCS XP analyser (Siemens Healthcare Diagnostics, Marburg, Germany), respectively. White blood cell, LY, NEUTRO, MO and BA counts were performed on a Sysmex XN-1000 analyser (Sysmex Corporation, Kobe, Japan) as a part of CBC. All the ratios were calculated by dividing counts of two types of cells, while dNLR was calculated by the formula dNLR = NEUTRO/(WBC – NEUTRO) [9] and M/GLR was calculated by the formula M/GLR = (WBC – LY)/LY [27].
Spirometry
Spirometry was used as a method of diagnosing the airflow limitation. It was performed on a Master-Screen Pneumo spirometer (Jaeger, Germany), according to the recommendations of the European Respiratory Society and American Thoracic Society. After three acceptable spirograms, airflow obstruction was confirmed in case of the ratio FEV1/FVC < 0.70. The protocol was performed as described before [37].
Therapy
Therapy data were collected from COPD patients. GOLD recommendations [2] about the therapy regimes were followed when COPD patients were subgrouped according to their treatment.
Statistical analysis
The Kolmogorov-Smirnov test was used for normal distribution testing. All data were non-parametric, so they were presented as median with interquartile range, while only age was presented as median with minimum and maximum. The χ2 test was used for comparison of males and females. Differences between controls and COPD patients were tested by the Mann-Whitney rank sum test, while the Kruskal-Wallis one way analysis of variance on rank test was used in the case of comparison between three or more groups of participants. Correlations were evaluated by the Spearman rank order test and obtained results were shown with the correlation coefficient (r) and p-value. Moreover, univariate logistic regression analysis was performed for evaluation of COPD predictors. Data were considered statistically significant if p < 0.05. Statistical analysis was performed by MedCalc statistical software, version 17.9.2. (MedCalc Software, Ostend, Belgium).
Results
Leukocyte subset counts and their ratios are altered in patients with stable chronic obstructive pulmonary disease
Basic characteristics, spirometry and common inflammatory parameters of all participants are shown in Table I. Chronic obstructive pulmonary disease patients and healthy subjects had similar age and sex distribution. As expected, lung function parameters were lower in COPD. In addition, systemic inflammation was present in COPD individuals, as verified by increased serum CRP and Fbg concentrations (p < 0.001). Table II shows the number of total circulating leukocytes and their subsets as well as their ratios in healthy and COPD individuals. White blood cells (p < 0.001), NEUTRO, MO and BA (p < 0.001) were higher in COPD patients than in the control group. Among calculated ratios, NLR (p < 0.001), dNLR (p < 0.001), M/GLR (p < 0.001), MLR (p < 0.001), BLR (p < 0.001) and BMR (p = 0.004) were increased. Other investigated parameters did not show any statistical significance (p > 0.05).
Table I
Basic characteristics, spirometry and common inflammatory markers of all participants included in the study
[i] Age is shown as median with minimum and maximum, and participants of each sex are shown as absolute numbers, while other data are shown as median with interquartile range. Comparison of males and females was performed by χ2 test, while other data were tested by Mann-Whitney rank sum test. Data were considered statistically significant when p < 0.05. n – number, COPD – chronic obstructive pulmonary disease, BMI – body mass index, FEV1 – forced expiratory volume in 1 s, FVC – forced vital capacity, CRP – C-reactive protein, Fbg – fibrinogen.
Table II
Leukocyte subsets and their ratios in control group and chronic obstructive pulmonary disease (COPD) patients
[i] Data are shown as median with interquartile range and tested by Mann-Whitney rank sum test. Results were statistically significant when p < 0.05. WBC – white blood cells, NEUTRO – neutrophil count, LY – lymphocyte count, MO – monocyte count, BA – basophil count, NLR – neutrophil to lymphocyte ratio, dNLR – derived neutrophil to lymphocyte ratio, M/GLR – monocyte/granulocyte to lymphocyte ratio, NMR – neutrophil to monocyte ratio, MLR – monocyte to lymphocyte ratio, BNR – basophil to neutrophil ratio, BLR – basophil to lymphocyte ratio, BMR – basophil to monocyte ratio.
Smoking effects on leukocyte-related parameters
We investigated the differences in leukocyte- related parameters in participants according to their smoking status (Table III). White blood cells were higher in healthy smokers than in healthy non-smokers as well as in COPD former smokers and COPD smokers in comparison to both healthy non-smokers and healthy smokers. There was no difference between healthy controls and COPD non-smokers. Moreover, NEUTRO was elevated in every COPD group when compared to healthy non-smokers, but COPD non-smokers were not significantly different in comparison to healthy smokers. Monocytes were elevated in both current smoking groups (healthy and COPD) as well as in COPD former smokers in comparison to healthy non-smokers, yet healthy smokers did not differ in comparison to COPD non-smokers. In addition, BA was elevated in COPD former smokers and COPD smokers when compared to healthy non-smokers and healthy smokers. Basophils, BLR and BMR were also increased in COPD smokers in comparison to COPD former smokers, while only BA showed a significant difference between COPD non-smokers and COPD smokers. Regarding other leukocyte parameters and ratios, we did not obtain any significant results when comparing healthy and COPD participants according to their smoking history (data not shown).
Table III
Differences in leukocyte parameters and ratios in healthy subjects and chronic obstructive pulmonary disease patients when subdivided into groups based on smoking status
| Parameter | Healthy non-smokers (n = 48) | Healthy smokers (n = 47) | COPD non-smokers (n = 5) | COPD former smokers (n = 75) | COPD smokers (n = 29) | P-value |
|---|---|---|---|---|---|---|
| WBC [× 109/l] | 5.46 (4.78–6.45) | 7.09 (5.84–7.89)1 | 6.65 (5.54–6.87) | 7.57 (6.63–8.91)1,2 | 8.21 (6.22–9.25)1,2 | < 0.001 |
| NEUTRO [× 109/l] | 3.22 (2.34–3.80) | 3.82 (3.05–4.57)1 | 4.41 (3.57–5.54)1 | 4.55 (3.74–5.73)1,2 | 5.24 (3.66–5.79)1,2 | < 0.001 |
| MO [× 109/l] | 0.47 (0.39–0.58) | 0.59 (0.47–0.73)1 | 0.58 (0.54–0.68) | 0.67 (0.56–0.89)1,2 | 0.63 (0.49–0.77)1 | < 0.001 |
| BA [× 109/l] | 0.020 (0.020–0.035) | 0.030 (0.020–0.048) | 0.040 (0.025–0.043) | 0.040 (0.030–0.060)1,2 | 0.060 (0.040–0.063)1,2,3,4 | < 0.001 |
| BLR | 0.012 (0.010–0.020) | 0.013 (0.008–0.024) | 0.022 (0.015–0.035)1 | 0.022 (0.015–0.030)1,2 | 0.029 (0.022–0.034)1,2,4 | < 0.001 |
| BMR | 0.051 (0.040–0.071) | 0.053 (0.027–0.077) | 0.069 (0.037–0.089) | 0.059 (0.040–0.081) | 0.085 (0.065–0.108)1,2,4 | < 0.001 |
Chronic obstructive pulmonary disease severity affects neutrophil to lymphocyte ratio, derived neutrophil to lymphocyte ratio and monocyte/granulocyte to lymphocyte ratio in chronic obstructive pulmonary disease patients
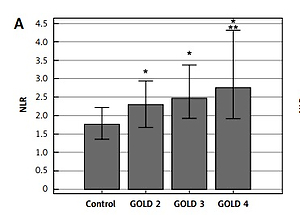
Differences in NLR, dNLR and M/GLR were obtained in COPD patients according to the grade of airflow obstruction as well as symptoms severity and exacerbation history (Figure 1). Ratios were higher in each FEV1-based (GOLD 2–4) stage in comparison to controls as well as in GOLD 4 in comparison to GOLD 2 stage (p < 0.05). In addition, dNLR was found to be increased in patients within GOLD B and GOLD D groups, while NLR and M/GLR were higher in each symptoms/exacerbation-based (GOLD A–D) group than in the healthy group of participants.
Figure 1
Neutrophil to lymphocyte ratio (NLR) (A), derived neutrophil to lymphocyte ratio (dNLR) (B) and monocyte/granulocyte to lymphocyte ratio (M/GLR) (C) in chronic obstructive pulmonary disease patients regarding GOLD 1–4 classification according to airflow obstruction severity and ABCD assessment based on symptoms severity and exacerbation history. *Statistically significant in comparison to controls. **Statistically significant in comparison to GOLD 2. All data are presented as median with interquartile range. Differences between GOLD 2–4 and GOLD A–D in comparison to control group were tested by Kruskal-Wallis one way analysis of variance on ranks. Data are considered statistically significant if p < 0.05, and post-hoc analysis was performed. Number of participants in each group was as follows: n (control) = 95; n (GOLD 2) = 39; n (GOLD 3) = 36; n (GOLD 4) = 34; n (GOLD A) = 14; n (GOLD B) = 63; n (GOLD D) = 32
Association of inflammatory parameters with lung function, dyspnoea and multicomponent chronic obstructive pulmonary disease indices
Results of Spearman’s rank correlation test showed no correlations between well-established inflammatory parameters (CRP and Fbg) and COPD-relevant parameters such as FEV1/FVC, BODCAT, DOSE and mMRC (data not shown). However, NLR, dNLR, M/GLR, and MLR were found to be significantly correlated with FEV1/FVC (r = –0.335, p < 0.001; r = –0.316, p < 0.001; r = –0.342, p < 0.001; r = –0.294, p = 0.002, respectively), while NLR, dNLR and M/GLR also correlated with mMRC (r = 0.295, p = 0.002; r = 0.276, p = 0.004 and r = 0.291, p = 0.002, respectively). Finally, NLR and dNLR correlated with BODCAT (r = 0.251, p = 0.008; r = 0.250, p = 0.009, respectively) and DOSE (r = 0.260, p = 0.006; r = 0.257, p = 0.007, respectively).
Diagnostic value of inflammatory parameters
Univariate logistic regression analysis showed that among inflammatory parameters examined, dNLR had the highest OR of 5.07, while OR of NLR was 2.86 and OR of M/GLR was 2.60 (p < 0.001) (Table IV).
Table IV
Univariate logistic regression analysis of inflammatory parameters
Differences in leukocyte subsets and ratios according to common therapy
Leukocyte-related parameters were also investigated in COPD patients subdivided according to their chronic inhalation therapy in four groups. They were taking bronchodilators only or in combination with ICS. Still, no statistically significant differences were found between the treatment groups (data not shown).
Discussion
In this study, certain leukocyte subsets (NEUTRO, MO, BA) and their ratios (NLR, dNLR, MLR, BLR, BMR, M/GLR) were higher in COPD patients than in age- and sex-matched healthy subjects, and some of them were associated with disease severity and smoking history.
Regarding cigarette smoke as being one of the COPD developmental contributors, it was reported that WBC, NEUTRO, LY, MO and BA might be increased in healthy smokers, but their numbers normalize after 5 years of smoking cessation [38, 39]. On the other hand, despite smoking cessation, there is ongoing inflammation in lung tissue in COPD patients due to the production of cytokines [40]. However, those effects are not necessarily restricted to the pulmonary compartment, and concomitant inflammatory responses in the extrapulmonary, i.e. systemic, compartment might be present in COPD patients as well. Gumus et al. found an increase in NLR due to smoking [38] and it was observed that NLR may be elevated in patients with more severe COPD in comparison to early stages of the disease [41, 42]. Our study also suggested some alterations of leukocyte-related parameters regarding smoking in the healthy population and COPD. Increased NEUTRO and MO were observed in healthy smokers compared to healthy non-smokers, suggesting that those leukocyte subtypes are the most affected by smoking. Interestingly, there were no differences in NEUTRO and MO between healthy smokers and COPD non-smokers, which may be due to a common underlying mechanism, i.e. smoke-derived oxidants trigger redox imbalance leading to oxidative stress, a condition that is also a part of COPD pathogenesis. Still, the number of COPD non-smokers was too low to test this assumption. Evaluation of BA, BLR and BMR in our study showed significantly higher values in COPD smokers in comparison to COPD former smokers. To the best of our knowledge, our study is the first presenting basophil-related parameters in accordance with smoking and/or COPD.
Due to the inflammation, the presence of inflammatory cells in the airways is broadly reflected by increased numbers of the same cells in peripheral blood. It was reported that airway obstruction and even a rapid decline in lung function were associated with increased number of neutrophils in sputum [43]. Therefore, since there is a correlation between sputum and blood neutrophils, COPD inflammation was also characterized by an increased number of neutrophils in peripheral circulation, and this was associated with COPD progression [44]. Peripheral blood monocytes are the precursors of tissue macrophages, which are implicated in the pathogenesis of COPD together with neutrophils. Similar to neutrophils, it was shown that monocytes were related to the COPD symptoms and reduced FEV1 [33]. In our study, when neutrophils and monocytes were combined in relevant ratios, NLR, dNLR, M/GLR and MLR were elevated in patients with COPD. dNLR and M/GLR were established as modified NLR parameters and we aimed to assess their performances in stable COPD due to the already known role of NLR in inflammation [16]. In a recent review paper, Pascual-Gonzalez et al. stated that there are conflicting results about the role of NLR in FEV1-based disease severity since some of the authors did not manage to show the relationship between NLR and GOLD stages, while only one study showed differences in NLR throughout GOLD stages in stable COPD patients [40]. However, we showed that NLR, dNLR and M/GLR successfully distinguished very severe COPD (GOLD 4) from moderate disease stage (GOLD 2). On the other hand, regarding symptoms severity, dNLR was similar in the control group and COPD group with less severe symptoms (GOLD A), while NLR and M/GLR were already increased in GOLD A compared to healthy individuals. To the best of our knowledge, dNLR and M/GLR were not associated with COPD severity and were not compared to NLR in COPD before. Lee et al. and Furutate et al. showed positive associations of NLR with mMRC and BODE index [23, 24]. Similar findings were reported in our study regarding the association of NLR and dNLR with lung function, dyspnoea, BODCAT and DOSE as well as the association of M/GLR with lung function and dyspnoea in COPD patients. In addition, all aforementioned parameters suggested a potential predictive role in COPD. Data about parameters associated with airflow obstruction and symptoms severity might help in disease assessment and improvement of the therapy approach. In their large review of clinical outcomes in COPD Paliogiannis et al. suggested that evaluation of COPD therapies is lacking [28]. Therefore, we assessed a possible therapy-related alteration in leukocyte subsets and their calculated ratios. However, bronchodilators alone or in combination with ICS did not cause significant changes in those parameters. Thus, it seems that leukocytes are not significantly affected by common COPD therapy. Nevertheless, further studies should be encouraged with a larger number of COPD patients subdivided into specific therapy groups.
Although we presented some novel and interesting results, especially those related to disease severity, therapy regimes and comparison of NLR, dNLR and M/GLR, our study has some limitations. It is a cross-sectional and case-control study, and it did not encompass COPD patients of GOLD 1 stage or those in the GOLD C group. Therefore, it would be useful to assess the parameters from our study in the early stage of the disease and clarify whether they might differ from the healthy population. In addition, a larger number of all participants should be considered in the further studies, especially considering participants in groups regarding different classification criteria as well as multicentre design of the studies.
In conclusion, many common parameters assessed from peripheral blood in a daily laboratory routine have a potential in COPD assessment due to their fast and easy determination, availability and already existing well-established methods. Among those are leukocyte subsets and their ratios, and we confirmed their role in ongoing systemic inflammation in COPD. In addition, we found that increased numbers of neutrophils, monocytes and basophils in COPD patients could be only partly explained by smoking, while COPD underlying pathophysiological mechanisms often include oxidative stress as well. NLR, dNLR and M/GLR were associated with COPD severity and distinguished very severe COPD patients from the moderate stage of the disease. They also showed a similar association with lung function and dyspnoea. Still, there were some differences in their associations with symptoms severity and exacerbation history (ABCD classification) as well as with multicomponent BODCAT and DOSE indices. Finally, NLR, dNLR and M/GLR were found to be associated with COPD, and could have a potential in identifying COPD patients, with dNLR having the highest OR of 5.07. Therefore, we believe that NLR, dNLR and/or M/GLR might become simple but helpful ancillary parameters in the assessment of COPD and some other inflammatory diseases.