Introduction
Antiphospholipid antibodies (aPL) constitute a heterogenous family of immunoglobulins reacting with a variety of plasma proteins. Among them lupus anticoagulant (LA), anticardiolipin antibodies (aCL), and anti-β2 glycoprotein I (aβ2GPI) are those best known. Their elevated plasma level is critical for antiphospholipid syndrome diagnosis [1] and was included in systemic lupus erythematosus (SLE) diagnostic criteria as well [2]. There is some evidence that in coronary artery disease (CAD) patients aPL remain within the normal range but are significantly higher than in healthy individuals [3, 4]. Their prothrombotic activity in venous and arterial thrombosis was well documented [5], but their action in atherosclerosis progression is still not clear [6]. Robust evidence for the impact of aPL on atherosclerosis progression comes from peripheral arterial disease (PAD) studies. On the other hand, in CAD aPL are associated with the occurrence of myocardial infarction (MI) rather than CAD progression itself [6].
Among endothelial dysfunction markers (EDM) C-reactive protein (CRP) is highly associated with intensification of inflammation, with thrombosis promotion [7] and thus with coronary plaque instability [8]. Although CRP is not recommended for routine cardiovascular (CV) risk refinement assessment [9], it is sensitive and it reflects different mechanisms of atherosclerosis [7]. Intracellular adhesion molecule-1 (ICAM-1) is a glycoprotein facilitating diapedesis across the vessel wall and, similarly to CRP, correlates with endothelial activation and damage [7]. sICAM-1 is a soluble form of ICAM-1 and is regulated by the same mechanisms as ICAM-1 [10]. Von Willebrand factor (vWF) also reflects endothelial injury and plays a relevant role in platelet activation and aggregation [11]. Because it is a nonspecific marker its role may be limited to accessory function with other biomarkers. Despite the fact that those biomarkers may be used to assess atherosclerosis activity in CAD patients, they probably might be also useful in monitoring atherosclerosis in SLE patients.
Statin’s role is substantial in CAD treatment and has been well described in numerous studies [12, 13]. The major, dose-dependent effect of treatment is low-density lipoprotein (LDL) cholesterol lowering through inhibiting hepatic cholesterol synthesis. Statins also decrease triglyceride serum level and increase high-density lipoprotein (HDL) cholesterol serum level, but this effect is achievable with higher doses. Intensive treatment may reduce the volume of coronary artery atherosclerotic plaques and change their composition [13, 14]. Statin pleiotropic action is based on isoprenoid inhibition and influences numerous signaling pathways. In vivo studies showed an effect on nitric oxide synthesis, production of proinflammatory cytokines, vasoconstrictive factors and reactivity of platelets [15–18]. These may result in a reduction of inflammation [19, 20] and a decrease in the thrombosis occurrence [21].
In SLE, statin treatment is still debated. Although it has been confirmed that statins reduce the risk of thrombosis [22], may have a similar effect on atherosclerosis as in CAD restraining atherosclerotic plaque progression [23] and reduce CV risk [24], they are not used routinely in current pharmacotherapy [25]. Even though currently CAD and atherosclerosis are the leading causes of death in SLE, statin treatment is restricted to patients with known hypercholesterolemia and/or symptomatic CAD [26].
Our objective was to investigate the influence of statin treatment on autoimmunity assessed by aPL levels and endothelial damage/activation measured by sICAM-1 and vWF levels in young CAD or SLE patients, as two models of autoimmune-dependent atherosclerosis.
Material and methods
Patients
Fifty-eight patients were enrolled in the study. Forty patients (mean age: 38.9 (±4.6), 35 male) with confirmed angiographically CAD underwent a myocardial revascularization procedure in the Department of Cardiac and Vascular Diseases, Jagiellonian University, John Paul II Hospital in Krakow between January 2012 and December 2014. Among 18 patients with SLE (mean age: 38.8 ±11.9, 1 male) treated in the Department of Internal Medicine, Jagiellonian University, Krakow (January 2010 – December 2014) 11 were treated with standard pharmacotherapy (9 on methylprednisolone (≤ 4 mg/day), 1 on chloroquine derivate, 1 on azathioprine). Precise study group characteristics is depicted in Table I.
Table I
Study subgroup characteristics
After obtaining written, informed consent, conforming to the International Declaration of Helsinki, patients were enrolled in the study. Exclusion criteria in the CAD group were: age at diagnosis above 45 years old or severe organ failure (including renal disease – grade 4 and 5 KIDGO, heart failure NYHA IV, respiratory failure). Patients with known autoimmune disease were excluded as well.
Subjects with SLE were enrolled only if in stable clinical condition and without known other autoimmune disease, including antiphospholipid syndrome (APS). A documented period of at least 3 months of steady doses of immunosuppressants was obligatory. Exclusion criteria concerning organ failure were the same as in the CAD group. Systemic lupus erythematosus activity index (SLEDAI) score at baseline (BL) was within the range 3–20, median 4. After a year the median SLEDAI score value remained at the same level (4) but the range of results was different (0–20). In 5 patients there was a decrease in SLEDAI score, in 5 an increase and in 8 the SLEDAI score remained at the same level.
Statin treatment
Both groups of patients were treated with atorvastatin for 1 year. Dose of the statin depended on BL LDL level in the CAD group; the treatment target was established at 1.8 mmol/l, and it was achieved in 90% of patients. Mean dose of atorvastatin was 46.1 ±22.2 mg. In 22 CAD patients previous treatment with low dose statin (not reaching the target LDL value) was documented. In SLE patients a fixed dose of 40 mg of atorvastatin was administered (no patients with hypercholesterolemia).
Laboratory tests
All patients were tested for aPL (LA, aCL, aβ2GPI) and endothelial dysfunction markers: sICAM-1, vWF:Ag as well as hsCRP at BL and after 1 year of atorvastatin treatment.
Antiphospholipid antibodies were measured in blood serum according to the local laboratory standard: aCL and aβ2GPI in IgG and IgM classes with home-made enzyme-linked immunosorbent assay (ELISA). Sapporo standard (murine monoclonal antibodies against HCAL for IgG and EY2C for IgM) was used to measure aβ2GPI. Cut-off values for aPL were as follows: aCL IgG 10 GPL, aCL IgM 20 MPL, aβ2GPI IgG 20 SGU, aβ2GPI IgM 20 SGU, and reflected the 99th percentile of the healthy population. Detection of LA was performed according to three-step analysis recommended by the International Society on Thrombosis and Haemostasis (ISTH). Only a negative test of LA was considered normal.
Soluble form of intracellular adhesion molecule-1 levels were assessed with a quantitative immunoenzymatic assay (ELISA) kit (R&D, BIOKOM, UK) in blood serum. Normal values for sICAM-1 were in the range 98.8–320 ng/ml. Measurements of vWF:Ag were done in plasma with commercial ELISA kit (Hyphen, Austria), normal values: 50–160%. C-reactive protein was assessed in serum, with the commercial analyzer BN II System and dedicated reagents (Siemens, Germany). Normal values for CRP: 0–5 mg/l.
Samples in the CAD group at BL were taken at least 4 weeks after the coronary procedure (mean: 15.5 weeks). Time between samples collection, BL and follow-up, was at least 12 months (mean: 74 weeks). Samples in the SLE group were taken within 1 week after enrollment of a patient in the study. Time between samples collection (BL to follow-up) was at least 12 months (mean: 63 weeks). Before laboratory analysis was performed samples were stored in the central laboratory refrigerator at –70°C (from collection until transportation to the central laboratory stored at –20°C, for 2–3 weeks).
Statistical analysis
Statistical analysis was performed using Statistica 10, Sigma Software. Numerical data were presented using mean value ± standard deviation (SD) or median value and range (min-max) in case of lack of normal distribution. Categorical variables were presented as a proportion. Differences in continuous variables were assessed with the Wilcoxon signed-rank test in case of repeated measurements or if no normal distribution (Student’s t-test in case of normal distribution). Categorical variables were compared using the χ2 test. The p-value for statistical significance was established at < 0.05.
Results
In the whole study population none of the patients fulfilled revised criteria for APS [1] within the observational period.
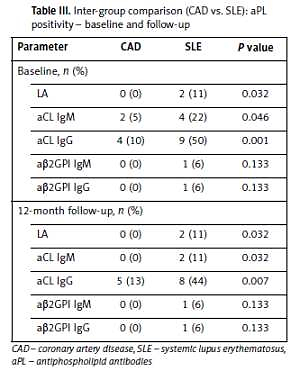
At BL, aβ2GPI IgG and IgM were higher in CAD patients, whereas aCL IgG and vWF:Ag were higher in SLE patients (Table II). The percentage of patient with out-of-range values of aCL in IgG and IgM classes and with LA positivity was higher in SLE patients at BL, as well as at 1-year follow-up (Table III). Only 2 patients exceeded the level of 40 GPL and/or SGU (aCL, aβ2GPI) during the whole study period, both in the SLE group.
Table II
Inter-group comparison (CAD vs. SLE): aPL, sICAM-1, vWF:Ag, CRP serum levels – baseline and follow-up
Table III
Inter-group comparison (CAD vs. SLE): aPL positivity – baseline and follow-up
After 12 months of treatment with atorvastatin in the CAD study subgroup there was a significant decrease of aβ2GPI IgG and of CRP serum levels. There was also a statistically significant increase in aCL IgG, sICAM-1 and vWF:Ag (Table IV). Contrary to these changes, in the SLE subgroup there was a decrease in CRP level only, whereas sICAM-1, vWF:Ag and aPL remained at similar levels.
Table IV
Comparison within CAD and SLE groups: aPL, sICAM -1, vWF:Ag, CRP serum levels – baseline and follow-up
Discussion
Although CAD in young patients in the majority of cases is connected with conventional risk factors [27], in approximately 20% it may be a result of coronary abnormalities and non-traditional risk factors such as connective tissue disorders, drugs and autoimmunity [28]. Some studies show that aPL serum levels are higher in young CAD patients than in healthy controls [3, 4]. In SLE patients autoimmunity is confirmed to contribute to atherosclerosis progression not only due to increased risk of thrombosis, but also by plaque progression [5, 23].
This paper shows that BL aPL, although within the normal range, are higher in the CAD than in the SLE group. This seems unusual, as aPL are a part of SLE diagnostic criteria [2]. Moreover, it seems that atorvastatin has a weaker effect on aPL in SLE compared to CAD, as there was no decrease of aβ2GPI in the SLE group. Initial measurements were done in treated, immunologically stable SLE patients (median SLEDAI score 4), which may be relevant in the context of aPL level. Recently Serrano et al. [29] reported that immunosuppression may reduce aβ2GPI: in post-renal transplant patients elevated aβ2GPI IgA level before surgery was significantly decreased after immunosuppressant introduction.
The main finding is that statin decreases aβ2GPI IgG in CAD. Among aPL, aβ2GPI are more often, as compared to aCL/ LA, associated with CAD and may reflect disease severity and future outcomes [30]. Moreover, aβ2GPI serum level elevation was proven to influence atherosclerosis plaque progression and subclinical atherosclerosis via T cell response [31] and promote oxLDL accumulation in macrophages [6] apart from its thrombotic activity. Clinical manifestation of elevated aβ2GPI is associated with future revascularization extent [30]. In many trials aCL and LA did not correlate with CAD progression or symptoms [32, 33]. In younger population (< 50 years old) however, aCL may have more clinical relevance [4, 34]. No direct comparison of younger and older populations has been done yet. Single studies showed increased risk of recurrent MI and stent restenosis [4, 35] in patients with elevated aCL serum levels, but there is no proven mechanism of atherosclerosis progression other than influencing thrombosis.
Another interesting observation of this study is the way statins affect endothelial damage. In some animal model studies [36] fluvastatin did decrease tissue factor (TF) and sICAM-1 level. Another possible molecular mechanism may be that suggested by Zheng et al. in an animal model, connecting statin lipoprotein associated phospholipase A2 inhibition with decrease of endothelial dysfunction [37]. In our study an increase of sICAM-1 level in the atorvastatin treated CAD arm was observed. The differences between individual statin action, or insufficient dose of statin in the CAD population (despite the decrease of CRP an sICAM-1 rise was observed) might be the cause of that fact. What may confirm this thesis is the fact that conventional risk factors present in the CAD study population, may continuously damage endothelium and cause sICAM-1 elevation. The interpretation of vWF:Ag level rise after atorvastatin treatment may be similar to that related to sICAM-1 values, although this marker is more vulnerable to other factors [11].
Statin-related CRP reduction was observed in both arms. That reflects a significant decrease of inflammation. The mechanism, proven to be independent of LDL lowering [22, 38], was associated with the reduction of atherosclerotic plaque volume, but may not be sufficient to stop atherosclerosis progression.
In conclusion, in clinically stable patients IgM and IgG class aβ2GPI levels are higher in CAD than in SLE, whereas IgG class aCL are higher in SLE. Statin treatment decreases the CRP level in both CAD and SLE patients. Moreover, atorvastatin decreases the aβ2GPI IgG level in CAD patients. The observed rise of aCL IgG, vWF:Ag and sICAM -1 in CAD during statin treatment may reflect atherosclerosis progression due to traditional risk factors or an insufficient dose of statin.