Introduction
Preeclampsia (PE) is a type of hypertension specific to human pregnancy, with an incidence of 2% to 7%. PE is primarily clinically manifested as an increase in urinary protein and blood pressure after 20 weeks of gestation [1], and represents one of the main etiologies for maternal and perinatal morbidity and mortality. The high-risk factors for PE include primiparity age over 40 years, obesity, diabetes mellitus, assisted reproductive conception, a family history of PE, multiple pregnancies, a previous pregnancy of PE and chronic hypertension [2]. To date, the mechanisms of pathogenesis underlying PE remain unclear. MicroRNAs (miRNAs) are a group of single-stranded non-coding small RNA molecules that are 18–23 nucleotides in length. Accumulating studies in recent years have shown that some miRNAs are abnormally expressed when PE occurs; one of them is miR-155 [3–5]. Abnormal expression levels of miRNA-155 may be closely associated with the development of such dysfunctions as trophoblast invasion and migration [6], whereas the specific molecular mechanisms still warrant more explorations and studies. As a G protein-coupled receptor, CXCR4 is expressed in the villus tissue throughout pregnancy. After binding to its ligand, CXCR4 can activate the downstream PI3K/AKT signaling pathway to affect the expression levels of genes within cells, thereby regulating the invasion and migration of the trophoblasts within the human villus tissues in early pregnancy [7, 8].
Salvianolic acid B (Sal B) is an aqueous bioactive component in Salvia miltiorrhiza, and a polyphenolic compound highly abundant within this plant. Sal B has the molecular formula of C36H30O16, an important water-soluble active ingredient in Salvia miltiorrhiza, and possesses most of the biological activity of Salvia miltiorrhiza [9]. Studies have shown that Sal B has been extensively used in various diseases, such as angina pectoris, pulmonary fibrosis and malignancies [10–12]. Sal B is most commonly used for treatment of cardiovascular disease, such as myocardial infarction and heart failure [9, 13, 14]; however, the roles of Sal B in improving PE and related mechanisms underlying it remain elusive.
Herein, we for the first time explored the efficacy of Sal B in PE through in vivo experiments, and initially investigated the roles of miRNA-155/CXCR4 in it; then, we validated the relevant mechanisms of action through cell experiments. By doing so, we investigated the mechanisms of action of Sal B in improving PE, and provided a solid experimental basis for the clinical treatment of PE.
Material and methods
Materials
Sal B was purchased from MCE (MedChemExpress, USA), and endotoxin from Sigma, USA, with a purity of 99.9%; the NO kit was a product from Shanghai Yanjing Biotechnology Co., Ltd., early pregnancy human extravillous trophoblast HTR8/SVneo from the Cell bank, Chinese Academy of Science, and fetal bovine serum and RPMI1640 medium from Gibco, USA. miR-155 mimics, negative control (NC), miRNA-155 inhibitor and miRNA-155 inhibitor negative control (miRNA-155 inhibitor NC) were designed and synthesized by Shanghai Gene Pharma Co., Ltd., China. The sequences of miRNA-155 mimics (5′ to 3′) were: sense: UUAUGCUAAUCGUGAUAGGGGU; antisense: CCCUAUCACGAUUAGCAUUAAUU; the sequence of miRNA-155 inhibitor (5′ to 3′) was ACCCCUAUCACGAUUAGCAUUAA; the sequences of NC (5′ to 3′) were: sense: UUCUCCGAACGUGUCACGUTT; antisense: ACGUGACACGUUCGGAGAATT; and the sequence of miRNA-155 inhibitor NC (5′ to 3′) was CAGUACUUUUGUGUAGUACAA. The following materials were used: RPMI1640 medium (Hyclone), Opti-MEM medium (Gibco), fetal bovine serum (FBS, Hangzhou Evergreen), trypsin (Geneview), transfection reagent Lipofectamine 2000 and Trizol (Invitrogen), RNA reverse transcription kit, SYBR green fluorescence Dye (Toyobo), Real-time PCR System, Transwell Chamber (BD Biosciences), protein concentration assay kit (Beijing CW Century Co., Ltd.), protein marker molecular marker (Geneview), rabbit anti-human CXCR4 monoclonal antibody, rabbit anti-human p-AKT monoclonal antibody, and rat anti-human β-actin monoclonal antibody (Abcam).
Experimental methods
Establishment and grouping of PE models
SD rats of clean grade with a body weight of 200–250 g were fed in a standard clean environment. The experiment was conducted in strict accordance with the National Institutes of Health (NIH) guidelines for feeding and use of laboratory animals. The male and female rats were housed in separate cages at a ratio of 2 : 1, with the finding of a vaginal plug as the 1st day of pregnancy. A total of 60 female rats were randomized to 5 groups, with 12 rats in each group. In addition to the normal group, the rats in the drug groups (low-, medium-, and high-dose groups) and the model control group were intravenously instilled with endotoxin 1.0 μg/(kg·d) at 2 ml/h at the 14th day of pregnancy for 4 days, and the rats in the blank control group were given normal saline of an equal dose. The conditions for H/R were hypoxia at 37°C with 2% O2 and 5% CO2 for 2 h, and reoxygenation at 37°C with 20% O2 and 5% CO2 for 6 h [15].
Methods of administration
From the 14th to the 21st day of pregnancy, the rats in the low-, medium- and high-dose drug groups were intraperitoneally injected with different concentrations of Sal B injection (20.0, 40.0, and 80.0 mg/kg), and the rats in the blank control and model control groups were administered normal saline of an equal dose until the 21st day of pregnancy.
Indicators for detection
The blood pressure of the tail artery, the content of 24 h urine protein and the concentration of plasma NO were monitored on the 19th and 21st days of pregnancy, respectively. 1) Test of blood pressure of the tail artery: the blood pressure of the rats’ tail artery was measured by means of the multi-channel non-invasive pressure measurement system. The rat was fixed in an incubator at 30°C for about 10 min, and the probe was fixed. The blood flow was blocked by the pressure increased by the tail compression sleeve until the blood flow of the tail artery had been blocked and the pulse wave had disappeared. After 3 to 5 s, the software was turned on and the external pressure was gradually decreased until the arterial pulsation appeared, at which time the blood pressure was recorded as systolic blood pressure of the experimental animal. Each rat was measured repeatedly 6 times at an interval of 2 min, with the mean value used. 2) Content of 24 h urine protein: the 24 h urine of the rat was collected, and the concentration of urine protein was determined through colorimetry. 3) Concentration of the plasma NO: 5 ml of blood from the abdominal aorta was collected and centrifuged, with the supernatant taken for use. The absorbance value of the supernatant was measured using the nitrate reductase method and converted to the concentration of NO.
HE staining
The placental tissues were removed, partially fixed in 4% paraformaldehyde for 24 h, embedded in paraffin, and sectioned (4 μm). The placental tissues underwent HE staining for pathological examination.
Total RNA extraction, reverse transcription and amplification
The placental tissues or cells were taken out from the rats and subjected to extraction of total RNA using the Trizol method, and the concentrations and purity of RNA in all groups were determined. 2 μg of RNA was reverse transcribed into cDNA, with the volume of the reverse transcription system being 20 μl. Amplification was performed after reverse transcription, with 3 replicate wells set in each group. Primers were synthesized by Shanghai Sangon Biotechnology Co., Ltd., and the primer sequences were as follows: CXCR-4 F: 5′-ACTACACCGAGGAAATGGGCT-3′; R: 5′-CCCACAATGCCAGTTAAGAAGA-3′; β-actin F: 5′-GGGAAATCGTGCGTGACATTAAGG-3′; R: 5′-CAGGAAGGAAGGCTGGAAGAGTG-3′. miRNA-155 F: 5′-TTAATGCTAATCGTGACT-3′; R: 5′-ACCTGAGAGTAGACCAGA-3′. U6 F: 5′-CTCGCTTCGGCAGCACA-3′; R: 5′-AACGCTTCACGAATTTGCGT-3′. The amplification procedure was pre-denaturation at 95°C for 10 min; 95°C for 15 s; 60°C for 1 min (40 cycles in total); prolongation at 72°C for 10 min. The relative expression levels of target genes were used as the criterion, and the relative expression levels of CXCR4 and miRNA-155 mRNA were calculated using 2–ΔΔCt.
Cell culture, grouping and treatment
The RPMI1640 cell culture medium containing 10% fetal bovine serum was added to the HTR8/SVneo cells and cultured in a constant-temperature incubator at 37°C with 5% CO2 and 95% saturated humidity. The cells were divided into the NC, H/R, BL, miRNA-155 mimics, miRNA-NC, inhibitor NC, miRNA-155 inhibitor, Sal B and Sal B + miRNA-155 groups.
Western blot
The fresh placenta tissues or cells from each group were placed, washed with pre-cooled PBS 3 times and then 50 μl of protein lysate (containing protease inhibitor and phosphatase inhibitor at 1 : 100) added to make the protein solution, whose concentration was determined using the BCA method. Protein loading was 80 μg, and 12% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) was prepared by means of electrophoresis for separation of proteins, followed by membrane transfer. CXCR4 was blocked with 5% skim milk powder, and p-AKT with 5% BSA at room temperature for 2 h, after which the blocking solution was discarded. Primary antibody CXCR4 (diluted at 1 : 500), p-AKT (diluted at 1 : 5 000) and internal reference β-actin (diluted at 15,000) antibody were added, incubated at 4°C overnight, secondary antibody (diluted at 1 : 1 : 1000) was added, and it was incubated at room temperature for 2 h, protected from light. The ODYSSEY Clx detection and imaging system was used for scanning and photographing.
Transwell cell invasion test
Prior to seeding of cells, it was necessary to equilibrate the transwell chamber and the 24-well plate: 100 μl of serum-free medium and 600 μl of FBS were added to the upper and lower chamber, respectively, and placed at 37°C with 5% CO2 overnight. The cells in all the groups transfected and starved for 24 h were collected, counted, inoculated into the upper chamber at 2 × 104 cells/well, and cultured at 37°C with 5% CO2 for 36 h. Then, the cells in the upper chamber were erased, and those in the lower chamber were fixed in methanol, and photographed after Giemsa staining. A total of 5 fields of view were used under high power microscope for counting, with the mean value used. The procedure for each group was repeated in triplicate.
Wound healing experiment
The cells underwent the scratch test at 24 h after transfection. After the medium in the six-well plate had been replaced with serum-free medium for 12 h starvation of cells, a “hyphen”-shaped scratch was created with a 200 μl pipette tip along the plate to form a central blank area. The tip was washed with PBS once, and the scratch area was photographed (0 h) under a microscope. Another 24 h culture was performed, and observation and photography (24 h) were done. The relative migration distance of cells was analyzed using Image J software. The experiment was repeated in triplicate, with the mean value used.
Cellular immunofluorescence
After the cells in each group were treated for 48 h, the medium was discarded. The cells were washed with pre-cooled PBS, fixed in 2 ml of 4% paraformaldehyde for 20 min, permeabilized and blocked with 1% polyethylene glycol octylphenyl ether, to which rabbit anti-human p-AKT monoclonal antibody (1 : 100) was added, and incubated in a wet box at 4°C overnight. To the cells were then added FITC-labeled goat anti-rabbit fluorescent secondary antibody (diluted at 1 : 50, American Arthox, USA) dropwise, and incubated at 37°C for 1 h. The nuclei were stained with propidium iodide (PI) and the sections were sealed with glycerin. The colored locations and the intensity of fluorescence were observed under a laser confocal microscope.
Statistical analysis
Statistical analysis was performed with the SPSS 20.0 statistical software. The experimental data in this study were represented by mean ± SD. One-way analysis of variance was adopted for inter-group comparisons, and the SNK-q test for multiple inter-group comparisons. The test level was α = 0.05.
Results
Histopathological effects of Sal B on placenta
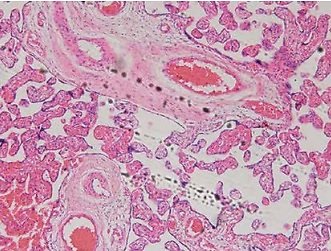
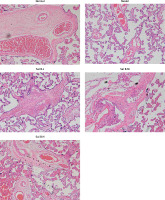
Trophoblastic nodules and fibrinoid necrosis were rare in the placenta in the NC group, capillaries were abundant in number, and vascular syncytium cell membranes were well formed. Marked nucleus vacuolarization of the syncytiotrophoblast cells was seen in the placental tissue in the model group, together with a large number of syncytial nodules, fibrinoid necrosis, and a decreased number of microvessels in the villi. After the administration of Sal B, occasional spiral arteries and some syncytial nodules were seen in the Sal B group, and constantly improved with the increase in the concentration of Sal B. See Figure 1 for details.
Figure 1
Pathology of different groups by HE staining (×100). Normal – the rats were treated by normal treatment; Model – the rats were treated by PE model; Sal B-L – the rats were treated by low-dose Sal (20.0 mg/kg) based on PE model; Sal B-M – the rats were treated by medium-dose Sal (40.0 mg/kg) based on PE model; Sal H-H – the rats were treated by high-dose Sal (80.0 mg/kg) based on PE model
Effects of Sal B on systolic blood pressure and urine protein concentration in PE rats
After induction with endotoxin drugs (D19), the systolic blood pressure and urine protein of rats in the model group and other drug groups were significantly higher than in the blank control group (p < 0.05), suggestive of the successful establishment of the model. Model establishment between the low-, medium- and high-dose drug groups and the model group was comparable. At 4 days after the intervention of the various drug groups with the different concentrations of Sal B, the systolic blood pressure in each drug group was significantly lower than in the model group (p < 0.05), and with the increase in the drug dose, the decrease in the systolic blood pressure was more obvious and the systolic blood pressure in the high-dose group was significantly lower than in the low-dose group (p < 0.05). The concentration of urine protein in various drug groups was lower than in the model group, with the difference being statistically significant (p < 0.05). The content of urine protein also showed a dose-dependent manner in different drug groups. After drug intervention, the concentrations of plasma NO in rats in various drug groups were generally higher than those prior to drug intervention, and significantly higher than in the model group (p < 0.05, Table I).
Table I
Comparison of systolic blood pressure and urine protein concentration in preeclampsia rats of different points
| Group | N | SBP [mm Hg] | 24 h proteinuria [mg/l] | NO [mmol/l] | |||
|---|---|---|---|---|---|---|---|
| D19 | D21 | D19 | D21 | D19 | D21 | ||
| Normal | 12 | 113.7 ±4.2 | 115.8 ±3.5 | 142.8 ±22.6 | 140.5 ±22.5 | 154.8 ±28.8 | 153.4 ±33.5 |
| Model | 12 | 139.6 ±3.8* | 138.5 ±4.2* | 351.6 ±42.9* | 360.5 ±50.9* | 52.8 ±9.4* | 54.6 ±8.8* |
| Sal B-L (20.0 mg/kg) | 12 | 140.2 ±4.1* | 128.1 ±3.8*Δ# | 361.5 ±52.9* | 208.5 ±35.9* | 62.8 ±9.8* | 72.8 ±10.8*Δ# |
| Sal B-M (40.0 mg/kg) | 12 | 139.7 ±4.0* | 125.2 ±4.0Δ | 355.8 ±48.5* | 175.4 ±9.5* | 58.4 ±8.9* | 97.6 ±15.7*Δ# |
| Sal B-H (80.0 mg/kg) | 12 | 139.5 ±4.8* | 116.4 ±4.5Δ | 359.8 ±55.4* | 153.7 ±10.5* | 57.5 ±8.7* | 130.5 ±20.6*Δ# |
Effects of Sal B on placental weight, body weight and body length of newborn rats
The placental weight, body weight and body length of the newborn rats in the model group were significantly lower than in the blank control group (p < 0.05). The placental weight, body weight and body length of the newborn rats in the medium- and high-dose groups were significantly higher than in the model group (p < 0.05). The placental weight, body weight and body length of the newborn rats in the low-dose group were higher than in the model control group, but the difference was not statistically significant (Table II).
Table II
Placental weight, neonatal rats’ weight and neonatal rats’ body length in different groups
| Group | N | Placental weight [g] | Neonatal rats’ weight [g] | Neonatal rats’ body length [cm] |
|---|---|---|---|---|
| Normal | 12 | 0.73 ±0.13 | 5.24 ±0.55 | 3.89 ±0.35 |
| Model | 12 | 0.48 ±0.12 | 4.36 ±0.41* | 3.12 ±0.35 |
| Sal B-L (20.0 mg/kg) | 12 | 0.51 ±0.14*# | 4.56 ±0.49*# | 3.24 ±0.40*# |
| Sal B-M (40.0 mg/kg) | 12 | 0.61 ±0.13*Δ | 4.78 ±0.60*Δ | 3.30 ±0.41Δ |
| Sal B-H (80.0 mg/kg) | 12 | 0.66 ±0.15Δ | 5.00 ±0.54*Δ | 3.62 ±0.35Δ |
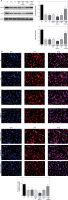
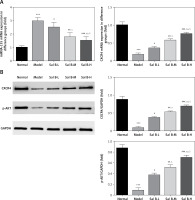
Effects of Sal B on miRNA-155, CXCR4 and p-AKT in the placenta of PE rats
RT-PCR quantitative analysis showed that compared with the normal group, the expression of the miRNA-155 gene was significantly increased while that of the CXCR4 gene was significantly decreased (p < 0.01, Figure 2 A) in the model group. Compared with the model group, the expression of the miRNA-155 gene was significantly inhibited while that of the CXCR4 gene was significantly elevated (p < 0.01, Figure 2 A) in the drug groups, with significant differences seen between the drug groups (p < 0.05, Figure 2 A). Semi-quantitative WB assay showed that compared with the normal group, the expression levels of the CXCR4 and p-AKT proteins in the model group were significantly lower (p < 0.01, Figure 2 B); compared with the model group, the expression levels of the CXCR4 and p-AKT proteins in the drug groups were significantly higher (p < 0.01, Figure 2 B), with significant differences seen between the drug groups (p < 0.05, Figure 2 B).
Figure 2
miRNA-155 and CXCR4 mRNA by RT-PCR and CXCR4 and p-AKT protein expression by WB assay. Normal – the rats were treated by normal treatment; Model – the rats were treated by PE model; Sal B-L – the rats were treated by low-dose Sal (20.0 mg/kg) based on PE model; Sal B-M – the rats were treated by medium-dose Sal (40.0 mg/kg) based on PE model; Sal H-H – the rats were treated by high-dose Sal (80.0 mg/kg) based on PE model. A – miRNA-155 and CXCR4 mRNA expression by RT-PCR assay. B – CXCR4 and p-AKT protein expression by WB assay
***P < 0.001, compared with normal group; #p < 0.05, ##p < 0.01, ###p < 0.001, compared with model group; Δp < 0.05, ΔΔp < 0.01, compared with Sal B-L group, $p < 0.05, compared with Sal B-M group.
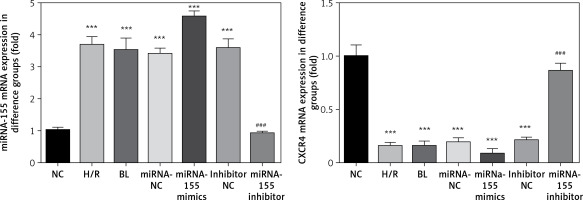
Expression of miRNA-155 and CXCR4 genes in cells in various groups
Compared with the NC group, the expression of the miRNA-155 gene in H/R, BL, miRNA-155 mimics, miRNA-NC and inhibitor NC groups was significantly higher while the expression of the CXCR4 gene was significantly lower (p < 0.001, Figure 3). However, compared with the H/R group, the expression of the miRNA-155 gene in the miRNA-inhibitor group was significantly lower while the expression of the CXCR4 gene was significantly higher (p < 0.001, Figure 3).
Effects of miRNA-155 on invasion and migration of cells
The transwell cell invasion and wound healing experiments showed that compared with the NC group, the number of transmembrane cells and the rate of wound healing were significantly lower in the H/R, BL, miRNA-155 mimics, miRNA-NC and inhibitor NC groups (p < 0.001, Figure 4), and that compared with the H/R group, the number of transmembrane cells and the rate of wound healing were significantly higher in the miRNA-inhibitor group (p < 0.001, Figure 4).
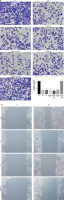
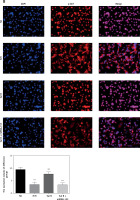
Effects of miRNA-155 on related proteins and entry of p-AKT into the nucleus
Compared with the NC group, the expression of the CXCR4 and p-AKT proteins and the amount of p-AKT protein entering the nucleus in the H/R, BL, miRNA-155 mimics, miRNA-NC and inhibitor NC groups were significantly lower (p < 0.001, Figure 5). However, compared with the H/R group, the expression of the CXCR4 and p-AKT proteins and the amount of p-AKT protein entering the nucleus were significantly higher in the miRNA-inhibitor group (p < 0.001, Figure 5).
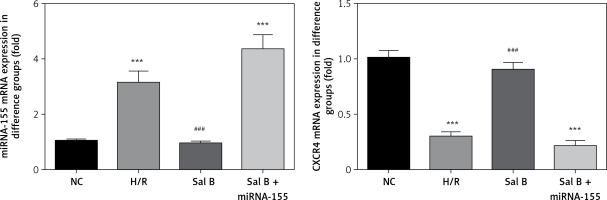
Effects of Sal B on expression levels of miRNA-155 and CXCR4 genes in cells in various groups
Compared with the NC group, the expression of the miRNA-155 gene was significantly higher in the H/R group and Sal B + miRNA-155 group, while the expression of the CXCR4 gene was significantly lower (p < 0.001, Figure 6). However, compared with the H/R group, the expression of the miRNA-155 gene was significantly lower in the Sal group, while the expression of the CXCR4 gene was significantly higher (p < 0.001, Figure 6).
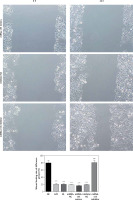
Effects of Sal B on invasion and migration of cells
The transwell cell invasion and wound healing experiments showed that compared with the NC group, the number of transmembrane cells and the rate of wound healing were significantly lower in the H/R group and the Sal B + miRNA-155 group (p < 0.001, Figure 7); compared with the H/R group, the number of transmembrane cells and the rate of wound healing in the Sal B group were markedly higher (p < 0.001, Figure 7).
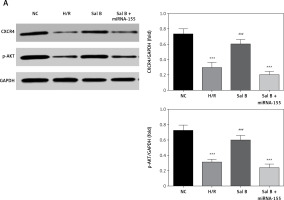
Effects of Sal B on related proteins and entry of p-AKT into the nucleus
Compared with the NC group, the expression of CXCR4 and p-AKT proteins and the amount of p-AKT protein entering the nucleus in the H/R group and the Sal B + miRNA-155 group were significantly lower (p < 0.001, Figure 8); however, compared with the H/R group, the expression of CXCR4 and p-AKT proteins and the amount of p-AKT protein entering the nucleus in the Sal group were significantly higher (p < 0.001, Figure 8).
Discussion
PE represents an idiopathic hypertensive syndrome presenting with hypertension and proteinuria, etc. after 20 weeks of gestation in women with normal blood pressure. It can lead to serious maternal complications and is one of the main causes of maternal and perinatal death [16]. The incidence of PE in Europe and the United States at present is low (approximately 2–5%), while it is more common in developing countries, with an incidence of about 10%. PE accounts for 9% of maternal mortality in Asia and Africa [17]. Among 10 million pregnant women worldwide every year, gestational hypertension, especially PE, leads to the death of 76,000 pregnant women and 500,000 fetuses or neonates [18]. The American College of Cardiology has also included PE among the risk factors for cardiovascular disease, and recommended that patients with a history of PE be understood and their lifestyles be improved [19, 20]. However, the mechanisms of pathogenesis underlying PE remain unclear, and there is still a lack of effective treatment options in clinical practice. Pregnancy has to be terminated early in many cases, causing pregnancy failure, and many epidemiological studies have also confirmed that the development of PE may have long-term adverse consequences in the offspring [21, 22]. The mechanisms of pathogenesis underlying PE may involve many factors, such as placental ischemia and hypoxia, inflammatory responses, changes in the PE-associated signaling pathways, trophoblastic infiltration abnormalities, abnormal maternal-fetal interface immunity, local coagulation of placenta and imbalanced anticoagulant mechanisms, and the abnormal expression profiles of miRNAs [23, 24].
Since the discovery of the first miRNA in Caenorhabditis elegans by Lee et al. in 1993 [25], more than 2,000 miRNAs have been discovered, and bioinformatics has predicted that miRNA may regulate 60% of genes encoding proteins within the human genome [26]. Great strides have been made in research on miRNAs in inflammation and tumors, while research on eclampsia-associated miRNAs has just started. miRNAs can regulate the post-translational expression of genes by undergoing complementary pairing with the 3′ non-coding region (3′UTR) of mRNA completely or incompletely, and plays an important role in the differentiation, development, proliferation, invasion, apoptosis and other biological processes of cells [27]. Encoded by the BIC gene, the miRNA-155 gene is located on human chromosome 21q21. 3, and is a miRNA closely associated with inflammation, tumors and immune regulation [28]. Recent studies have shown that miRNA-155 can target the regulation of expression of CXCR4, affect the proliferation and angiogenesis of cancer cells and increase the invasiveness of tumor cells in such diseases as gastric cancer and breast cancer [29, 30]. CXCR4, which is also known as chemokine receptor 4, is located on the human chromosome 2q21, and consists of 352 amino acids. It can be expressed on the membranes of various cells, including trophoblast cells, and is expressed in the villus tissues throughout pregnancy [31].
Sal B is an active ingredient from the traditional Chinese medicine Salvia miltiorrhiza. Pharmacological studies have shown that Sal B can regulate the vascular tone by blocking the β-receptors, reducing the calcium influx, antagonizing endothelin, and increasing the generation of NO in the platelets, etc., thus increasing the blood flow [32, 33]. These show that Sal B exhibits good effects in combating platelet aggregation, dilating small arteries, and improving microcirculation and hemorheology, and that it can improve the invasion and migration of chorionic trophoblast cells.
This study demonstrated that Sal B exhibited marked effects on improving the blood pressure and urine protein and promoting the development and maturation of young rats, suggestive of the good effects of Sal B in improving the vascular tension of the placenta and improving hypoxia and ischemia of cells. In the meantime, it was found that Sal B could lead to an increased concentration of NO in the plasma and enhanced invasion and migration of trophoblast cells. The cell experiments showed that Sal B could restore the injury of invasion and migration of the chorionic trophoblast cells as a result of simulation of the hypoxia model of PE. The mechanisms underlying this may be associated with promoted CXCR4 expression levels and p-AKT protein activity and an increased nuclear load through inhibition of miRNA-155.
In conclusion, this study, through in vivo and in vitro experiments, demonstrated that Sal B could improve the pathological changes in PE by inhibiting miRNA-155, and its mechanisms may include activation of the CXCR4 /PI3K/AKT signaling pathway to affect such biological functions of trophoblasts as invasion and migration.