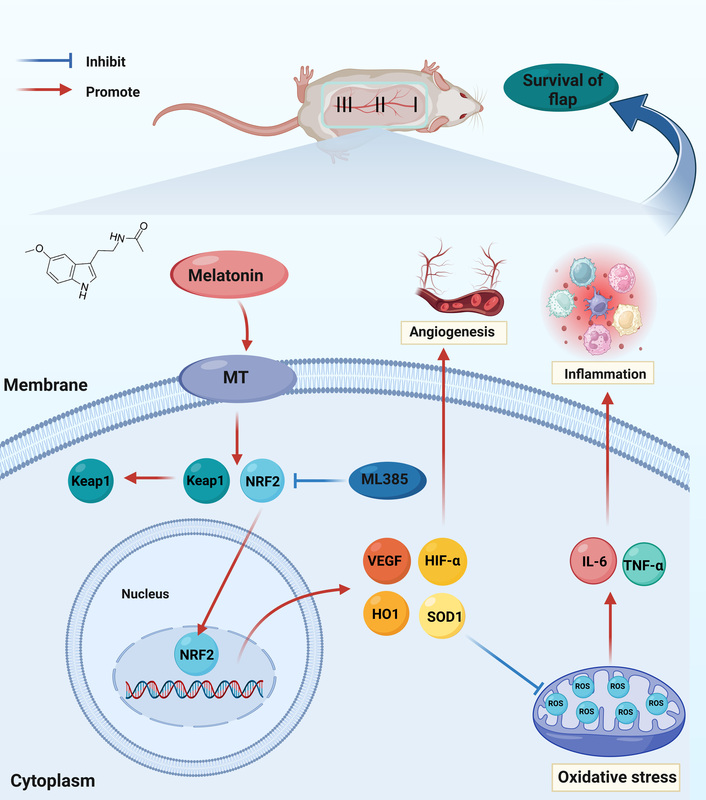
Introduction
Random skin flaps have been widely used in plastic surgery to repair and reconstruct skin defects caused by trauma, pressure ulcers, tumor resection, diabetic wounds, and other factors [1, 2]. The current treatment method is adversely affected by distal flap necrosis, limiting random skin flaps’ application. After transplantation, random skin flaps may undergo severe ischemia due to the lack of axial vessels, and then neovascularization initiates from the flap pedicle toward the distal part. Subsequently, partial restoration and reperfusion of blood supply can lead to ischemia-reperfusion injury (IRI) [3]. Recent studies have demonstrated that IRI-induced accumulation of reactive oxygen species (ROS) and inflammatory responses are important causes of skin flap necrosis [4, 5]. In response to the mechanism of ischemic necrosis, various methods have been developed to promote the survival of random skin flaps. These methods include stimulating angiogenesis, alleviating oxidative stress, and attenuating inflammation. Additionally, skin tissue engineering approaches based on novel hydrogels have also shown promising prospects [6, 7].
Nuclear factor erythroid-2-related factor 2 (NRF2) is a transcription factor that activates a series of antioxidant genes and protects against xenobiotic and oxidative stress [8]. Under stressful conditions, NRF2 dissociates from Kelch-like ECH-associated protein 1 (KEAP1) and translocates to the nucleus. Subsequently, NRF2 recognizes antioxidant response elements in the promoters of target genes, which include antioxidant enzymes and phase II detoxification enzymes, and triggers their expression [9]. ML385 is a small-molecule, NRF2-specific inhibitor that binds to NRF2 and interferes with the DNA binding activity of the NRF2-musculoaponeurotic fibrosarcoma oncogene homolog G (MAFG) protein complex, thereby inhibiting the expression of downstream target genes [10]. Since excessive ROS can induce inflammation and cell death, NRF2-mediated anti-inflammatory effects may be based on ROS elimination. Previous studies have shown that inflammatory responses in NRF2-deficient mice can be suppressed by inhibiting ROS production [11]. Furthermore, activation of the NRF2 antioxidant defense system is conducive to suppression of oxidative stress and promotion of random skin flap survival [12]. Altogether, these findings imply that drugs focused on modulating the NRF2 pathway could be an effective treatment for improving random skin flap survival.
Melatonin (MEL) is a pleiotropic hormone mainly synthesized and secreted by the pineal gland and is involved in various physiological functions, including antioxidant [13], anti-inflammatory [14], immunomodulatory [15], neuroprotection [16], circadian rhythm regulation [17], and oncostatic effects [18]. Notably, recent studies have demonstrated that melatonin not only attenuates inflammatory responses by scavenging ROS, but also induces activation of antioxidant enzymes via the NRF2 signaling pathway [19]. Furthermore, previous studies have shown that melatonin promotes angiogenesis by upregulating vascular endothelial growth factor (VEGF) levels during the progression of osteoporotic bone defect repair and gastric ulcer healing [20, 21]. Moreover, melatonin plays a protective role in IRI in many organs by reducing oxidative stress-induced cell damage [22, 23]. Although studies have shown that melatonin can alleviate ischemic necrosis of random skin flaps, the molecular mechanism remains unclear.
Therefore, we hypothesized that melatonin might stimulate angiogenesis, attenuate oxidative stress and inflammation through the NRF2 signaling pathway, and improve random skin flap survival. The aim of this study was to investigate the effect of melatonin on the survival of random skin flaps and to explore the related molecular mechanisms.
Material and methods
Animals
Male Sprague-Dawley (SD) rats weighing 200–250 g were purchased from the Laboratory Animal Center of our university (License No. SYXK [ZJ] 2020-0014). The rats were housed in single cages in an air-conditioned room with a temperature of 21–25°C, a humidity of 50–60%, and an alternating light/dark cycle every 12 h. All animal experiments were performed following the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health in China, with the approval of the Institutional Animal Care and Use Committee of our university (xmsq 2022-0057).
Reagents and antibodies
Melatonin (purity: 99.73%, cat# 73-31-4) and ML385 (purity: 99.72%, cat# 846557-71-9) were obtained from MedChemExpress. The HE stain kit (cat# G1120), DAB substrate kit (cat# DA1010), and goat serum (cat# SL038) were purchased from Solarbio Science & Technology. The DAPI Fluoromount-GTM (cat# 36308ES11) and dihydroethidium (DHE) (cat# 50102ES02) were purchased from Yeasen Biotechnology. The BCA protein assay kit (cat# P0010) was provided by Beyotime Biotechnology. The Omni-ECLTM enhanced pico light chemiluminescence kit and HRP-labeled goat anti-rabbit IgG (H + L) secondary antibody (cat# LF102) were obtained from Epizyme Biomedical Technology. The secondary antibodies of goat anti-rabbit IgG H&L (Alexa Fluor 488) (cat# ab150077) and goat anti-rabbit IgG H&L (Alexa Fluor 647) (ab150079) were acquired from Abcam. The primary antibodies against VEGF (cat# AF5131), hypoxia inducible factor 1α (HIF-1α) (cat# AF1009), superoxide dismutase 1 (SOD1) (cat# AF5198), heme oxygenase 1 (HO1) (cat# AF5393), and NRF2 (cat# AF0639) were purchased from Affinity Biosciences. Primary antibodies against cadherin5 (cat# A0734), matrix metalloproteinase 9 (MMP9) (cat# A0289), endothelial NOS (eNOS) (cat# A1548), interleukin 6 (IL-6) (cat# A0286), tumor necrosis factor α (TNF-α) (cat# A0277) and interleukin 1β (IL-1β) (cat# A16288) were purchased from Abclonal Technology. Primary antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (cat# BA2913) was provided by Boster Biological Technology. All general chemicals were of analytical grade and were purchased from Solarbio Science & Technology.
Random skin flap model
Rats were anesthetized with isoflurane (3% for induction and 2% for maintenance) using a gas anesthesia system. Dorsal hair was subsequently removed by shaving and applying depilatory cream. According to the modified McFarlane flap model [24], random skin flaps were established on the dorsal skin of rats. Briefly, a 9 cm × 3 cm rectangular area was drawn on the back of the rats with the midline as the long axis and the line connecting the iliac crests as the short side. Then, the skin was incised along the cranial and lateral lines of the rectangular area, and the bilateral iliac arteries were exposed and ligated. Afterward, the skin flap was immediately overlaid on the donor bed and sutured with 3-0 nylon single stitches. The skin flap was equally divided into three regions from the distal end to the pedicle: Area I, Area II, and Area III. In the random skin flap model, Area I exhibits necrosis, Area II suffers from ischemia and tends towards necrosis, while Area III shows normal health [25]. To improve the survival rate of the skin flap, the intervention aimed to inhibit ischemia and potential necrosis in Area II. Therefore, Area II was selected for examination to evaluate IRI and investigate factors that promote flap survival.
Experimental design and drug administration
A total of 72 rats were randomly separated into three groups: the Control group (n = 24), the MEL group (n = 24), and the MEL + ML385 group (n = 24). According to previous studies [26–28], the MEL group was given an intraperitoneal injection of melatonin (20 mg/kg/day) for 7 consecutive days after the procedure, the Control group was given the same amount of physiological saline, and the MEL + ML385 group was intraperitoneally injected with ML385 (30 mg/kg/day) 30 min before melatonin administration. On day 7 after surgery, all animals were euthanized with an overdose of pentobarbital sodium. Twelve rats in each group were used to evaluate the survival area, blood flow signal intensity, and tissue water content. Furthermore, samples from 6 rats per group were used for western blotting analysis, and samples from another 6 rats per group were used for immunohistochemistry staining, immunofluorescence staining, and HE staining.
Macroscopic assessment
After the procedure, the general condition of random skin flaps was observed daily, including skin color, appearance, texture, and hair growth. High-quality photographs of the skin flaps were taken on postoperative day 7. The necrotic area of the skin flap appeared black-brown, scabbed, tough, and without hair growth, while the surviving area was pink, supple, and had new hair growth. The digital photographs were analyzed with Image-Pro Plus software 6.0 (Media Cybernetics, USA), and the percentage of survival area was calculated as follows: percentage of survival area (%) = range of survival area/total area × 100%.
Tissue edema, another marker of skin flap necrosis, was analyzed by the tissue water content. On postoperative day 7 after the procedure, the skin flap tissue samples were weighed and recorded as wet weight. Subsequently, the samples were freeze-dried with a lyophilizer until the sample mass did not decrease within 2 days, and then the samples were weighed and recorded as dry weight. The percentage of tissue water content was calculated as follows: percentage of tissue water content (%) = (wet weight - dry weight)/wet weight × 100%.
Laser Doppler blood flow (LDBF) imaging
The blood flow of the skin flap was measured when the rats was under anesthesia and placed in an area delineated by the laser Doppler probe. The scanning was repeated three times. The signal intensity of blood flow in the skin flaps was quantified by MoorLDI software 6.1 (Moor Instruments, UK). The perfusion unit (PU) was calculated as the flow velocity multiplied by the concentration of red blood cells, as an indicator of blood perfusion.
HE staining
Six tissue samples of 1.0 cm × 1.0 cm were acquired from Area II of each flap, and immersed in 4% paraformaldehyde for 24 h. Then the samples were dehydrated, embedded in paraffin, and cut into 4 µm thick sections. The paraffin sections were stained with an HE stain kit. Subsequently, the number of microvessels was counted on six arbitrary fields per section under an optical microscope (Olympus Corporation, Japan), and the mean vessel density was determined as the average value of the number of microvessels per unit area (/mm2).
Immunohistochemistry
4% paraformaldehyde-fixed, paraffin-embedded, 4 µm thick tissue sections were first deparaffinized in xylene and rehydrated in a graded ethanol bath. The sections were incubated in 3% hydrogen peroxide, and antigen retrieval was carried out in 10.2 mM sodium citrate buffer for 20 min at 95°C. Sections were then washed with phosphate-buffered saline (PBS) and blocked with 10% goat serum for 10 min, and incubated with primary antibodies overnight at 4°C: HIF-1α (1 : 200), VEGF (1 : 200), TNF-α (1 : 200), IL-6 (1 : 200), NRF2 (1 : 200) and SOD1 (1 : 200). Sections were incubated with HRP-labeled secondary antibodies, and developed with a DAB substrate kit, followed with hematoxylin for counterstaining. Images were captured under an Olympus microscope with a DP2-TWAIN image acquisition software system (Olympus Corporation, Japan). Image-Pro Plus software 6.0 (Media Cybernetics, USA) was used to quantify the integral absorbances of HIF-1α, VEGF, TNF-α, IL-6, NRF2 and SOD1 positive vessels. Immunohistochemical analysis was performed on 6 random fields of 3 sections of each sample.
Immunofluorescence and DHE staining
The sections were deparaffinized and rehydrated as described above. After washing three times, tissue antigen was retrieved using sodium citrate buffer (20 min, 95°C). The sections were permeabilized with 0.1% Triton X-100 in PBS, and then incubated in 10% goat serum in PBS for 1 h at room temperature. Afterward, the sections were incubated overnight at 4°C with the primary antibodies against HIF-1α (1 : 200), VEGF (1 : 200), TNF-α (1 : 200), NRF2 (1 : 200), and SOD1 (1 : 200). Next, the sections were incubated with secondary antibodies for 1 h and counterstained with DAPI. Finally, images of positive cells were captured using a fluorescent microscope (Olympus, Japan) and analyzed using Image-Pro Plus software 6.0 (Media Cybernetics, USA). For DHE staining, tissue samples of Area II were dehydrated with 30% sucrose solution after washing with PBS, and then embedded in optimal cutting temperature (OCT) compound. The samples were then frozen at –80°C overnight and cut into 20 µm sections. Next, the tissue slides were incubated with DHE solution in PBS for 30 min at room temperature. After washing, the tissue slides were imaged using a fluorescent microscope as above.
Western blotting
The skin flap samples were lysed with RIPA buffer containing protease inhibitors and PMSF and total protein was collected after centrifugation. The protein concentration was determined by the BCA protein assay kit. Equal amounts of protein samples (60 µg) were electrophoresed on 12% sodium dodecyl sulfate-poly-acrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene fluoride (PVDF) membranes. After blocking with 5% nonfat milk (Tris-Buffered Saline with Tween-20 buffer) for 2 h at room temperature, the membranes were incubated with the corresponding diluted primary antibodies at 4°C overnight: VEGF (1 : 1000), MMP9 (1 : 1000), cadherin5 (1 : 1000), eNOS (1 : 1000), HO1 (1 : 1000), SOD1 (1 : 1000), TNF-α (1 : 1000), IL-6 (1 : 1000), IL-1β (1 : 1000), NRF2 (1 : 1000), GAPDH (1 : 1000). Subsequently, the membrane was incubated with secondary antibody at room temperature for 2 h. After being visualized by the Omni-ECLTM enhanced pico light chemiluminescence kit, the protein bands were analyzed using Image Lab 3.0 software (Bio-Rad, USA).
Statistical analysis
Statistical analysis was performed using SPSS version 26 (IBM, USA). The mean and standard deviation (mean ± SD) of the quantitative data were presented. Comparisons between two groups were conducted using the independent sample t test, and comparisons of three groups were performed by one-way analysis of variance (ANOVA). A p-value of less than 0.05 was considered significant.
Results
Melatonin promotes the survival of random skin flaps
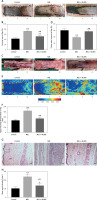
On postoperative day 7, we evaluated the activity of the random skin flap model by macroscopic assessment, tissue edema, LDBF imaging, and HE staining. Observation of the flap surface and subcutaneous region found that the ischemic necrosis area at the distal end of the flap appeared dark in color, hard, and shrunken, without hair growth, tissue edema, and severe congestion. In contrast, the surviving area presented light color, soft, and stretched, with hair growth, with less edema and congestion (Figures 1 A, C). Different degrees of ischemic necrosis appeared in Area I and II of the skin flaps in both groups, and the percentage of survival area of the flaps in the MEL group was higher than that in the control group (Figure 1 B). Moreover, the percentage of the tissue water content of flaps was significantly lower in the MEL group compared to the Control group (Figure 1 D). The LDBF imaging visualized the vascular network in the flap, and analysis of blood flow showed significantly stronger signal intensity in the MEL group than in the Control group (Figures 1 E, F). The analysis of HE staining revealed that the mean vessel density of flaps in the MEL group was significantly higher compared with that of the Control group (Figures 1 G, H). Collectively, these results suggest that melatonin promotes the survival of random skin flaps.
Figure 1
Melatonin promotes survival of random skin flaps. A – Digital photographs of random skin flaps in the Control and MEL groups were taken on postoperative day 7 (scale bar: 1.0 cm). B – Histogram of percentage of survival area on postoperative day 7. C – Digital photographs of tissue edema in the Control and MEL groups on postoperative day 7 (scale bar: 1.0 cm). D – Histogram of percentage of tissue water content. Data are presented as mean ± SD, n = 6 per group. E – Laser Doppler blood flow images of flaps showing vascular network and blood supply in the Control and MEL groups on postoperative day 7 (scale bar: 1.0 cm). F – Histogram of signal intensity of blood flow in flaps. G – HE staining in Area II of flaps showing the vessels in the Control and MEL groups (original magnification × 200) (scale bar: 50 μm). H – Histogram of mean vessel density calculated from HE staining. Data are presented as mean ± SD, n = 6 per group. *p < 0.05 and **p < 0.01, vs. Control group

Melatonin promotes angiogenesis in random skin flaps
Improvement in angiogenesis and restoration of blood supply are considered effective in promoting random skin flap survival. Therefore, to verify our hypothesis that melatonin enhances angiogenesis in random skin flaps, we performed immunofluorescence, immunohistochemistry, and western blot analysis. Expression levels of VEGF and HIF1α, two key regulators of hypoxia-induced angiogenesis, were assessed by immunofluorescence staining (Figures 2 A, B). The percentage of VEGF and HIF1α positive cells in the MEL group was significantly higher compared with the Control group (Figures 2 C, D). Moreover, the expression of VEGF and HIF-1α in the flaps was detected by immunohistochemistry, as shown in Figure 2 E. The results were consistent with the immunofluorescence staining, showing that the levels of VEGF and HIF1α in the MEL group were significantly higher than in the Control group (Figure 2 F). The expression levels of angiogenesis-related proteins such as VEGF, MMP9, and cadherin5 in Area II of random skin flaps were assessed by western blot analysis (Figure 2 G). The results showed that, compared with the Control group, the optical density values of angiogenesis-related proteins in the MEL group were all elevated (Figure 2 H). Therefore, these results indicate that melatonin enhances angiogenesis in random skin flaps.
Figure 2
Melatonin promotes angiogenesis in random skin flaps. A, B – Immunofluorescence staining for VEGF and HIF-1α positive cells in the dermal layer in the Control and MEL groups (scale bar: 20 μm). C, D – Histogram showing the percentages of VEGF and HIF-1α positive cells in the Control and MEL groups. Data are presented as mean ± SD, n = 6 per group. E – Immunohistochemistry for VEGF and HIF-1α expression in the flaps of the Control and MEL groups (original magnification × 200) (scale bar: 50 μm). F – Histogram showing the integral absorbance of VEGF and HIF-1α. G – Western blotting showing the expression of VEGF, MMP9 and cadherin5 in the Control and MEL groups. H – Histogram showing the optical density values of VEGF, MMP9 and cadherin5 in the Control and MEL groups. Data are presented as mean ± SD, n = 6 per group. *p < 0.05 and **p < 0.01, vs. Control group
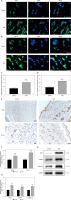
Melatonin reduces oxidative stress in random skin flaps
Oxidative stress, especially the accumulation of ROS, plays an important role in ischemia-reperfusion injury of skin flaps. Therefore, we hypothesized that melatonin promotes skin flap survival by reducing oxidative stress. To confirm our hypothesis, we assessed the level of ROS and the expression of antioxidant proteins such as SOD1, HO1, and eNOS in Area II of skin flaps. The ROS levels in the flaps were assessed by DHE fluorescence (Figure 3 A). Immunofluorescence assays showed that after treatment with melatonin, the optical density value of DHE was significantly lower than that of the Control group (Figure 3 C). Western blot analysis and immunofluorescence assays were performed to determine the expression of SOD1 in skin flaps (Figures 3 B, E). The results of both western blot and immunofluorescence showed that the expression of SOD1 in the MEL group was markedly higher than that in the Control group (Figures 3 D, F). HO1 is acknowledged as a cytoprotective enzyme due to its capacity to catabolize cytotoxic free heme and produce antioxidants [29]. The results of western blotting showed that the expression of antioxidant proteins (eNOS, HO1, and SOD1) was significantly higher in the MEL group than the Control group (Figures 3 G, H). Therefore, these results suggest that melatonin reduced oxidative stress in random skin flaps.
Figure 3
Melatonin reduces oxidative stress in random skin flaps. A, B – Immunofluorescence staining for ROS levels and SOD1 positive cells in the dermal layer in the Control and MEL groups (scale bar: 20 μm). C – Histogram showing the levels of ROS in the Control and MEL groups. D – Histogram showing the percentages of SOD1 positive cells in the Control and MEL groups. Data are presented as mean ± SD, n = 6 per group. E – Immunohistochemistry for SOD1 expression in the flaps of the Control and MEL groups (original magnification × 200) (scale bar: 50 μm). F – Histogram showing the integral absorbance of SOD1. G – Western blotting showing the expression of eNOS, HO1 and SOD1 in the Control and MEL groups. H – Histogram showing the optical density values of eNOS, HO1 and SOD1 in the Control and MEL groups. Data are presented as mean ± SD, n = 6 per group. *p < 0.05 and **p < 0.01, vs. Control group
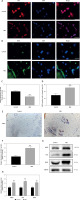
Melatonin alleviates inflammation in random skin flaps
Next, we investigated whether melatonin has a protective role in the inflammatory response of random skin flaps. First, immunofluorescence staining showed that melatonin significantly decreased the percentage of TNF-α positive cells in the dermal layer of the flaps (Figures 4 A, B). Further immunohistochemistry staining likewise showed that the expression levels of TNF-α and IL-6 in the MEL group were lower than those in the Control group (Figures 4 C, D). Moreover, western blotting results showed that melatonin administration decreased the expression of inflammatory factors (TNF-α, IL-6, and IL-1β) in the random skin flap model (Figures 4 E, F). Thus, these results suggested that melatonin alleviated inflammation by reducing inflammatory cytokine expression.
Figure 4
Melatonin alleviates inflammation in random skin flaps. A – Immunofluorescence staining for TNF-α positive cells in the dermal layer in the Control and MEL groups (scale bar: 20 μm). B – Histogram showing the percentages of TNF-α positive cells in the Control and MEL groups. C – Immunohistochemistry for TNF-α and IL-6 expression in the flaps of the Control and MEL groups (original magnification × 200) (scale bar: 50 μm). Data are presented as mean ± SD, n = 6 per group. D – Histogram showing the integral absorbance of TNF-α and IL-6. E – Western blotting showing the expression of TNF-α, IL-6 and IL-1β in the Control and MEL groups. F – Histogram showing the optical density values of TNF-α, IL-6 and IL-1β in the Control and MEL groups. Data are presented as mean ± SD, n = 6 per group. *p < 0.05 and **p < 0.01, vs. Control group
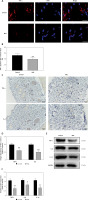
ML385 reverses the pro-survival effect of melatonin on random skin flaps
To investigate the role of NRF2 signaling in the therapeutic effect of melatonin on skin flap survival, we co-administered ML385 (a specific NRF2 inhibitor) with melatonin and evaluated the effects. The results indicated that the percentage of flap survival area in the MEL + ML385 group was significantly lower than that in the MEL group; meanwhile, the survival area was larger in the MEL + ML385 group than that in the Control group (Figures 5 A, B). Likewise, co-administration with ML385 aggravated tissue edema of flaps. The percentage of the tissue water content of the MEL + ML385 group was significantly higher than that of the MEL group (Figures 5 C, D). Moreover, the LDBF analysis showed that the signal intensity of blood flow of the MEL + ML385 group was significantly lower than that of the MEL group (Figures 5 E, F). Additionally, the analysis of HE staining demonstrated that the mean vessel density of flaps in the MEL + ML385 group was significantly lower than that in the MEL group (Figures 5 G, H). Altogether, these results suggested that ML385 significantly reversed the positive effect of melatonin on random skin flap survival, which may be associated with activation of the NRF2 signaling pathway by melatonin.
Figure 5
Inhibition of NRF2 signaling reverses the effects of melatonin on random skin flap survival. A – Digital photographs of flaps in the Control, MEL and MEL + ML385 groups were taken on postoperative day 7 (scale bar: 1.0 cm). B – Histogram of percentage of survival area on postoperative day 7. C – Digital photographs of tissue edema in the Control, MEL and MEL + ML385 groups on postoperative day 7 (scale bar: 1.0 cm). D – Histogram of percentage of tissue water content. E – Laser Doppler blood flow images of flaps showing vascular network and blood supply in the Control, MEL and MEL + ML385 groups on postoperative day 7 (scale bar: 1.0 cm). F – Histogram of signal intensity of blood flow in flaps. Data are presented as mean ± SD, n = 6 per group. *p < 0.05 and **p < 0.01, vs. Control group. G – HE staining in Area II of flaps showing the vessels in the Control, MEL and MEL + ML385 groups (original magnification × 200) (scale bar: 50 μm). H – Histogram of mean vessel density calculated from HE staining. Data are presented as mean ± SD, n = 6 per group. *p < 0.05 and **p < 0.01, vs. Control group. #p < 0.05 and ##p < 0.01, vs. MEL group
Melatonin promotes angiogenesis, reduces oxidative stress, and alleviates inflammation by activating NRF2
First, the results of immunohistochemistry and western blotting analysis showed that the level of NRF2 in the MEL + ML385 group was significantly lower than that in the MEL group (Figures 6 B, D, G, H). In addition, immunofluorescence staining results showed that the percentage of NRF2-positive cells in the flaps was significantly reduced in the MEL+ML385 group (Figure 6 A, C). These results indicated that NRF2 in melatonin-treated random skin flaps was successfully inhibited by ML385.
Figure 6
Inhibition of NRF2 signaling reverses the effects of melatonin on angiogenesis, oxidative stress and inflammation. A – Immunofluorescence staining for VEGF, SOD1, TNF-α, NRF2 and HIF-1α positive cells in the dermal layer in the Control, MEL and MEL + ML385 groups (scale bar: 20 μm). Data are presented as mean ± SD, n = 6 per group. *p < 0.05 and **p < 0.01, vs. Control group. #p < 0.05 and ##p < 0.01, vs. MEL group. B – Immunohistochemistry for VEGF, TNF-α and NRF2 expression in the flaps of the Control, MEL and MEL + ML385 groups (original magnification × 200) (scale bar: 50 μm). C – Histogram showing the percentages of VEGF, SOD1, TNF-α, NRF2 and HIF-1α positive cells in each group. D – Histogram showing the integral absorbance of VEGF, TNF-α and NRF2. E – Immunofluorescence staining for ROS levels in the dermal layer in each group (scale bar: 20 μm). F – Histogram showing the levels of ROS in each group. Data are presented as mean ± SD, n = 6 per group. *p < 0.05 and **p < 0.01, vs. Control group. #p < 0.05 and ##p < 0.01, vs. MEL group. G – Western blotting showing the expression of VEGF, SOD1, HO1, TNF-α, IL-6 and NRF2 in each groups. H – Histogram showing the optical density values of VEGF, SOD1, HO1, TNF-α, IL-6 and NRF2 in each group. Data are presented as mean ± SD, n = 6 per group. *p < 0.05 and **p < 0.01, vs. Control group. #p < 0.05 and ##p < 0.01, vs. MEL group
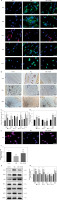
Subsequently, we assessed whether co-administration with ML385 affected the efficacy of melatonin in angiogenesis, oxidative stress, and inflammatory responses. The expression of proteins associated with angiogenesis, oxidative stress, and inflammation was determined using western blotting, immunohistochemistry, and immunofluorescence in random skin flaps. Firstly, immunofluorescence staining showed that, compared with the MEL group, the percentages of VEGF and SOD1 positive cells were significantly lower, while the percentage of TNF-α positive cells and the ROS level were significantly higher in the MEL + ML385 group (Figures 6 A, C, E, F). Moreover, immunohistochemistry staining showed that co-administration with ML385 significantly reduced the expression of VEGF and increased the expression of TNF-α (Figures 6 B, D). Similarly, the quantification results of western blotting showed that, compared with the MEL group, the levels of VEGF, SOD1, and HO1 were significantly lower, and the levels of inflammatory cytokines (TNF-α and IL-6) were significantly higher in the MEL + ML385 group (Figures 6 G, H). In summary, our findings suggested that melatonin activates NRF2 in random skin flaps, which is the primary mechanism by which melatonin promotes angiogenesis, reduces oxidative stress, alleviates inflammation, and ultimately improves the survival of random skin flaps.
Discussion
Among the skin flaps currently used in clinical practice, random skin flaps have become a common transplantation technique for repairing skin defects in plastic surgery because of their convenient sampling and similar color and texture to the skin at the wound site. The vascular supply of the random skin flap is through the dermal-subdermal plexus [30]. Therefore, the site and longitudinal axis of the donor flap are not restricted by the axial vessels. However, ischemic necrosis of random skin flaps is a common complication, especially in the distal portion of the flap. The main causes of ischemic necrosis of distal skin flaps are insufficient blood supply and ischemia-reperfusion injury. Oxidative stress and inflammatory response are two important mechanisms of ischemia-reperfusion injury, leading to further tissue damage or necrosis. The ischemic necrosis of distal flaps is an important reason for the poor prognosis of random skin flaps, including affecting the function and appearance of organs, limiting clinical application, prolonging hospital stay, and increasing the economic burden of patients [31]. Therefore, it is still of clinical significance to study strategies to improve the survival rate of random skin flaps.
Melatonin, an endogenous hormone secreted by the pineal gland, has the functions of regulating cell proliferation, differentiation, metastasis, metabolism, and apoptosis. The effect of melatonin on angiogenesis is different in different pathophysiological processes, which may be related to its specific regulation mechanism of melatonin receptor and VEGF. On the one hand, previous studies have shown that melatonin inhibits angiogenesis by suppressing the HIF-1α-VEGF pathway in vascular endothelial cells under hypoxia [32, 33]. On the other hand, it has been reported that melatonin promotes bone marrow mesenchymal stem cell (BMSC)-mediated angiogenesis in bone defects [20] and MMP2-mediated angiogenesis in gastric ulcers by upregulating the level of VEGF [21]. Furthermore, melatonin has been reported to attenuate inflammation, oxidative stress, and apoptosis by activating the NRF2 signaling pathway [34–36]. In addition, numerous studies have reported the protective effect of melatonin against ischemia-reperfusion injury in the brain, heart, liver, and other sites [37–39]. Therefore, we hypothesized that melatonin may reduce ischemic necrosis in random skin flaps, which was validated in our current work. Our study found that the survival area and tissue edema of random skin flaps improved, indicating that melatonin effectively promotes random skin flap survival.
Previous studies have shown that promoting angiogenesis is critical for flap survival [40]. Angiogenesis is a complex process of forming new blood vessels by sprouting endothelial cells from pre-existing blood vessels, including proliferation, differentiation, guided migration, and quiescence of endothelial cells [41]. VEGF serves as an initial angiogenic signal that promotes proliferation, migration and sprouting of endothelial cells, and increases vascular permeability [42]. HIF-1α initiates broad transcriptional responses to promote angiogenesis, through the upregulation of angiogenic factors such as VEGF [43]. In our study, we found that melatonin increased the signal density of blood flow in the flap and improved the vessel density in the dermis. Our results also showed increased expression levels of VEGF, HIF-1α, MMP9, and cadherin5 proteins with melatonin treatment, suggesting that melatonin promoted angiogenesis in random pattern skin flaps. Our study found that melatonin-induced angiogenesis stimulates the viability of random skin flaps.
Ischemia-reperfusion injury is an important cause of flap necrosis, manifested by the accumulation of ROS, inflammation, and apoptosis. High levels of ROS lead to oxidative stress, cellular damage and ultimately cell death. Previous studies have shown that melatonin acts as an antioxidant to thwart oxidative damage in a remarkable range of mechanisms [44]. Melatonin can not only directly scavenge a variety of ROS and reactive nitrogen species, but also indirectly stimulate antioxidant enzymes while inhibiting the activity of pro-oxidative enzymes. Therefore, we hypothesized that melatonin may promote random skin flap survival by inhibiting oxidative stress. SOD1 is an antioxidant enzyme that catalyzes the transformation of superoxide to hydrogen peroxide [45]. HO1 is a subtype of heme oxygenase with potent antioxidant activity. eNOS is an enzyme with antioxidant activity. Measurement of intracellular ROS was performed using DHE staining. In our study, we observed that melatonin increased the levels of anti-oxidative stress-related proteins SOD1, HO1, and eNOS and decreased the level of ROS in random skin flaps. These results suggest that melatonin promotes random skin flap survival by alleviating oxidative stress.
Inflammation has been implicated as one of the main responses in random skin flap ischemia-reperfusion injury, and blocking various aspects of the inflammatory cascade has been demonstrated to alleviate ischemia-reperfusion injury. Therefore, we hypothesized that melatonin may promote random skin flap survival by suppressing the inflammatory response. It was previously reported that melatonin ameliorated the progression of atherosclerosis through attenuating NLRP3 inflammasome activation [46]. Another study confirmed that melatonin effectively reduced the expression of pro-inflammatory factors TNF-α and IL-8 in severe osteoarthritis [47]. In our study, using western blotting, immunohistochemistry, and immunofluorescence, we observed that melatonin treatment reduced the levels of pro-inflammatory cytokines including TNF-α, IL-6, and IL-1β. These results suggest that melatonin promotes random skin flap survival by alleviating the inflammatory response.
ML385 is a specific NRF2 inhibitor which leads to a significant reduction in the expression of NRF2 and downstream target genes [48]. Furthermore, NRF2 signaling acts as a master regulator of antioxidant stress, regulating the expression of antioxidant genes and phase II detoxification enzymes such as HO1, which remove cytotoxic ROS to counteract oxidative damage [49]. The present study demonstrated that ML385 inhibits NRF2, thereby reversing melatonin-mediated promotion of skin flap viability and reduction of tissue edema. Furthermore, ML385 treatment reduced the levels of SOD1, HO1 and ROS in melatonin-treated flaps. These findings imply that melatonin alleviates oxidative stress in random skin flaps by activating NRF2. It has been reported that NRF2 suppresses the macrophage inflammatory response via blocking proinflammatory cytokine transcription [50]. Our study found that ML385 treatment suppressed the levels of TNF-α and IL-6 in random skin flaps treated with melatonin. This finding implies that melatonin alleviates inflammation in random skin flap through NRF2 activation. Previous studies have shown that NRF2 contributes to the angiogenic potential of endothelial cells [51]. NRF2 inhibition of cancer cells under hypoxic conditions results in the inability to accumulate HIF-1α protein, possibly due to reduced mitochondrial oxygen consumption [52]. In the present study, ML385 reduced the vessel density and vascular network distribution and inhibited the expression of VEGF in melatonin-treated flaps. This suggests that ML385 may attenuate angiogenesis by inhibiting NRF2. Therefore, our current research findings indicate that melatonin can activate NRF2 and facilitate its nuclear translocation, thereby enhancing the expression of antioxidant enzymes. The upregulation of these antioxidant enzymes inhibits oxidative stress and inflammation in random skin flaps, while also promoting angiogenesis.
Our study has some limitations that need to be addressed in future studies. First, our results are based on in vivo experiments, and no in vitro experiments were performed to determine other mechanisms by which melatonin enhances random skin flap survival. Second, this study used an effective dose of melatonin rather than concentration gradient to select an optimal dose. In addition, it is unknown whether the effects of melatonin on angiogenesis are affected by other conditions, such as drug concentration, timing, and duration of management. Whether other pathways participate in the antiangiogenic effect of melatonin requires further investigation. Furthermore, stem cell-based tissue engineering therapies are considered promising strategies for tissue regeneration, and future research can further explore the role of mesenchymal stem cells in skin tissue regeneration [53, 54]. Nevertheless, this study supports the benefit of melatonin for random skin flaps and lays the foundation for further research.
In conclusion, our study demonstrated that melatonin promotes the survival of random skin flaps by activating the NRF2 signaling pathway to promote angiogenesis and inhibit oxidative stress and inflammation. The results showed that inhibition of NRF2 reverses the beneficial effect of melatonin on random skin flaps. Activation of the NRF2 pathway may reduce ROS levels, thereby suppressing inflammation. Therefore, melatonin treatment enhances skin flap viability. The present work provides an important basis for evaluating the beneficial effects of melatonin on random skin flaps. Further studies should be conducted to better understand the potential clinical utility of melatonin.