Vitamin D deficiency is implicated in skeletal and non-skeletal disorders, including diabetes and cancers [1]. However, long-term follow-up studies [2] and randomized controlled trials (RCTs) assessing vitamin D supplementation for non-skeletal diseases have yielded inconsistent or negative results [3–7], casting doubt on a causal relationship.
Research on vitamin D’s pathogenic role, especially tumorigenesis, is ongoing. A recent mouse study demonstrated the anti-tumor effect of vitamin D [8]; reduced expression of 237 vitamin D receptor (VDR) target genes in five human cancers (skin cutaneous melanoma, sarcoma, hepatocellular carcinoma [HCC], breast and prostate cancer) correlated with poorer prognosis.
Vitamin D binding protein (VDBP) (Supplementary Table SI) is indispensable for transporting vitamin D and its metabolites, but its role in health and cancer remains controversial [9]. VDBP-deficient mice showed enhanced control of implanted tumors due to improved tissue-level vitamin D response [8], whereas a high level of VDBP was reported to exert anti-tumor effect in HCC [10].
MR analysis has become a commonly used method to explore whether there is a causal relationship between exposure and outcome, or between two disease states [11, 12]. Previous Mendelian randomization (MR) analysis found no causal link between circulating 25-hydroxyvitamin D (25OHD) and cardiovascular disease or cancer risk [13], but these did not assess other key vitamin D system components, such as tissue 25OHD availability regulated by VDBP and VDR activity indicated by target gene expression.
We conducted a comprehensive MR analysis evaluating causal effects of 25OHD, VDBP and VDR on the five cancers influenced by vitamin D [8]. This study aims to provide clear genetic evidence regarding the influence of 25OHD levels, tissue availability of vitamin D, and activity of the VDR on cancer risk. By elucidating these relationships, the study seeks to offer a potential explanation for why many clinical trials have struggled to replicate the promising results observed in basic research.
Methods
Study design.
A two-sample MR (TSMR) analysis was performed to examine the causal effects of serum 25OHD and VDBP on breast cancer (BRCA), melanoma, hepatocellular carcinoma (HCC), and prostate cancer (PCa), sarcoma (Supplementary Figure S1 A). Then SMR/TSMR analysis was used to examine the impact of VDR activity in blood and corresponding tissues on the tumorigenesis of these five cancers (Supplementary Figure S1 B). Sensitivity analysis assessed heterogeneity, potential horizontal pleiotropy and outliers.
Instrument and outcome selection
IVs of 25OHD were extracted from the GWAS genetic loci associated with serum 25OHD levels in 417,580 European UKB participants [14]. Of the 143 loci, we selected autosomal SNPs with p < 5 × 10–8. Palindromic SNPs, where alleles form complementary base pairs and complicate strand orientation, were excluded (n = 14), leaving 99 SNPs (Supplementary Table SII). F-statistics were calculated as β2/SE2.
IVs for VDBP were selected from a GWAS based on the Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH) [15] included 65,589 Danish neonates. Of the 26 SNPs identified, 3 palindromic SNPs were excluded, resulting in 23 SNPs (Supplementary Table SII). We conducted MR analysis using cis-pQTLs located within 1000 kilobases of the GC gene, which encodes VDBP.
A previous study identified 237 VDR target genes indicating VDR activation [8]. To evaluate their impact on cancers, eQTLs (Supplementary Table SIII) for these genes across 49 tissues from 838 donors were obtained from the GTEx project (V8), and peripheral blood eQTLs from eQTLGen (n = 31,684) [16]. Only cis-eQTLs-SNPs within 1 Mb of transcripts significantly associated with VDR gene expression (p < 5 × 10−8) were included, as this window captures key regulatory elements. Details are listed in Table I.
Table I
SMR and simulation analysis of eQTLs and five types of cancer
GWAS statistics for the five cancers were obtained from publicly databases: BRCA (BCAC, 122,977 cases; 105,974 controls), melanoma (FinnGen R10, 4,261 cases; 313,897 controls) [17], PCa (OncoArray, 79,148 cases; 61,106 controls), liver cancer (775 unrelated European patients with alcohol-related liver disease; 1,332 controls), and sarcoma (FinnGen R10, 191 cases; 313,897 controls). For replication MR of VDBP and sarcoma, UK Biobank data were used (235 cases; 456,041 controls). Details are in Supplementary Table SV.
Statistical analysis
For 25OHD and VDBP, causality was primarily estimated using the inverse variance weighted (IVW) method [18]. Robustness was checked with MR-Egger, weighted median, weighted mode, simple median, and MR-PRESSO tests. Horizontal pleiotropy was assessed via MR-Egger regression intercept, while MR-PRESSO identified outliers and recalculated causal estimates without them. Furthermore, leave-one-out analyses tested estimate stability, and Cochran’s Q statistic evaluated heterogeneity (p < 0.05 indicating significance). All palindromic SNPs were excluded to avoid strand ambiguity. Statistical analyses used TwoSampleMR/TSMR (V-0.6.4), MR-PRESSO (V-1.0), in R (V-4.3.0) [19], with visualization via ggplot2.
SMR analysis with HEIDI test for VDR target genes was conducted using SMR software (v1.3.1) with default settings (https://yanglab.westlake.edu.cn/software/smr/#Overview). A HEIDI p > 0.05 suggests the association is unlikely due to linkage disequilibrium [20]. To assess chance associations, 1,000 simulations randomly selected matching gene numbers per tissue; results were significant if observed exceeded ≥ 950 simulations. Corresponding probabilities were calculated with the phyper function in R.
Results
IV selection and validation
99 SNPs associated serum 25OHD and 23 SNPs associated with VDBP are used as instruments in TSMR analysis (Supplementary Tables SII, SIV). F-statistics of the IVs were all greater than 30, indicating the weak possibility of weak instrumental bias.
The causal relationships between serum 25OHD and cancers
IVW, MR Egger, weighted median, simple mode, and weighted mode method consistently showed no evidence of a causal relationship between serum 25OHD and breast cancer (IVW: odds ratio [OR] = 1.032, 95% CI = 0.890–1.197, p = 0.674), melanoma (IVW: OR = 1.077, 95% CI = 0.925–1.255, p = 0.340), HCC (IVW: OR = 1.100, 95% CI = 0.658–1.836, p = 0.717), prostate cancer (IVW: OR = 1.026, 95% CI = 0.856–1.230, p = 0.783), and sarcoma (IVW: OR = 0.876, 95% CI = 0.453–1.692, p = 0.693) (Figure 1). Scatter plots are presented in Supplementary Figure S2.
Figure 1
Mendelian randomization results of serum 25-hydroxyvitamin D (25OHD) as exposure and five types of cancer as outcome. This figure presents the Mendelian randomization analysis results evaluating the causal association between genetically predicted 25OHD levels and the risk of five cancer types: breast cancer, melanoma, hepatocellular carcinoma, prostate cancer, and sarcoma. For each cancer type, five different MR methods are applied, showing the number of instrumental SNPs, p-values, odds ratios (OR), and 95% confidence intervals (CI). Forest plots visualize the effect estimates and their precision relative to the null hypothesis of no effect (the 95%CI of OR includes 1), providing a comprehensive comparison of the potential influence of 25OHD on cancer susceptibility across analytical approaches

Heterogeneity test showed moderate heterogeneity for breast cancer (IVW Q p = 0.002) and prostate cancer (IVW Q p = 0.001) (Supplementary Table SVI). MR-Egger intercept indicated no horizontal pleiotropy, and leave-one-out analysis showed no single IV significantly influenced results (Supplementary Figures S3–S7). MR-PRESSO identified an outlier SNP (rs10908465) in breast cancer analysis, but its exclusion did not alter the null results. Funnel plots are shown in Supplementary Figure S8.
The causal relationship between VDBP and cancers.
Circulating VDBP binds 25OHD with highest affinity, regulating vitamin D availability to tissues [21]. MR results found no causal effect of VDBP on breast cancer (IVW OR = 1.015, 95% CI = 0.995–1.036, p = 0.135), melanoma (OR = 1.013, 95% CI = 0.987–1.040, p = 0.323), HCC (OR = 1.043, 95% CI = 0.961–1.133, p = 0.314), prostate cancer (OR = 1.003, 95% CI = 0.992–1.014, p = 0.583). However, higher VDBP was significantly associated with increased sarcoma risk (OR = 1.190, 95% CI = 1.054–1.344, p = 0.005) (Figure 2). UK biobank replication yielded null results (OR = 1.016, 95% CI = 0.902–1.145, p = 0.794) (Supplementary Figure S9). Scatter plots are shown in Supplementary Figure S10. The results of the analysis with cis-pQTLs consistently showed no significant associations between genetically predicted VDBP levels and the risks of five different types of cancers (Supplementary Figures S11–S15).
Figure 2
Mendelian randomization results of serum vitamin D binding protein (VDBP) as exposure and five types of cancer as outcome. This figure presents the Mendelian randomization analysis investigating the causal effects of serum vitamin D binding protein (VDBP) levels on the risk of five cancer types: breast cancer, melanoma, hepatocellular carcinoma, prostate cancer, and sarcoma. For each cancer, five MR methods are applied with the corresponding number of instrumental SNPs, p-values, odds ratios (OR), and 95% confidence intervals (CI) reported. Forest plots visually depict the effect estimates relative to the null value (the 95% CI of OR includes 1), enabling comparison of results across methods and cancer types to evaluate potential associations of genetically predicted VDBP levels with cancer susceptibility
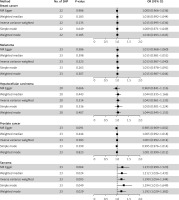
Heterogeneity test did not support existence of heterogeneity across analysis of VDBP and cancers (Q p > 0.05) (Supplementary Table SVII). The MR-Egger intercept indicated horizontal pleiotropy in the VDBP-prostate cancer analysis (MR Egger intercept = 0.012, p = 0.016) (Supplementary Table SVII). Leave-one-out and MR-PRESSO analysis detected no influential outliers (Supplementary Figures S16–S20). Funnel plots are shown in Supplementary Figure S21.
The causal relationship between VDR target genes and cancers.
SMR/TSMR analysis demonstrated that expression of most (90.91–97.22%) VDR target genes in breast, prostate, liver, skin tissues was not associated with breast cancer, prostate cancer, HCC, and melanoma risk, respectively (Supplementary Tables SVIII–SXII). Tissue-specific SMR for sarcoma was not feasible due to its heterogeneous origin. Peripheral blood analysis identified 139, 151, 145, 160 VDR target genes passing the HEIDI test per cancer type (Supplementary Tables SXIII–SXVII), with 87.42–96.25% showing no causal association. Among genes significantly associated with cancer (p_SMR < 0.05), effect direction was inconsistent. Simulation and hypergeometric analysis showed the number of significant eQTLs of VDR did not exceed chance expectations (all p > 0.05) (Table I).
Discussion
This study found no significant evidence supporting causal associations between serum 25OHD levels, VDBP or VDR activity and risks of BRCA, PCa, HCC, melanoma and sarcoma.
The anti-tumor effects of vitamin D are well documented in humans and rodents [22]. Vitamin D exerts its effects mainly through its active form, 1,25-dihydroxyvitamin D3 (calcitriol), which binds to the VDR, a nuclear receptor that regulates gene transcription. Its anti-tumor effects have been observed in various cancers, including breast, prostate, colon, glioblastoma, and others, suggesting it plays a versatile role in suppressing tumor development and progression through these molecular mechanisms (Supplementary Figure S22). Mechanisms include inhibition of tumor cell proliferation, dedifferentiation, invasion and metastasis [22, 23]; regulation of cancer cell metabolism; protection against oxidative stress [24]; immune modulation within tumor microenvironments [24]. It is thus of interest to examine whether all these findings at molecular and cellular levels can translate into the causal association between vitamin D and cancers.
However, nearly all MR analyses, including ours, consistently find no significant associations between genetically predicted higher serum 25OHD levels and cancer risk [25]. Our study further revealed neither VDBP nor VDR activity affects cancer risks. This divergence from experimental evidence warrants discussion.
Observational studies are prone to confounding (e.g., diet, lifestyle, sun exposure) and reverse causation, such as high-risk individuals altering vitamin D intake. MR uses genetic variants as instruments to infer causality, minimizing such biases, but limited power can yield null results. In this study, all IVs had F-statistics > 30, surpassing the threshold for instrument strength. We focused on five cancers from recent research, but still found no causal link between 25OHD and cancer risk.
The relationship between vitamin D and tumor risk might be nonlinear, with protective effects evident only at specific thresholds. Observational studies might capture these nuances, whereas MR assumes linearity, potentially obscuring them. Some studies indicate vitamin D’s preventive effects manifest only at low 25OHD levels [26]. Thus, evidence for vitamin D status impacting extra-skeletal outcomes is lacking. Observational studies capture long-term supplementation effects, influencing tumor risk, whereas MR reflects lifelong genetic predisposition, which might not parallel the effects of short-term supplementation.
Serum 25OHD, tightly bound to VDBP, reflects vitamin D status, while tissue availability is mainly regulated by VDBP. Global knockout of VDBP in mice enhanced anti-cancer immunity despite lower circulating 25OHD, suggesting increased tissue redistribution [8]. This prompted further MR analysis of VDBP and cancer risk. Although elevated VDBP was linked to higher sarcoma risk, replication analysis was inconsistent.
A mouse study showed vitamin D exerted effects on glucose intolerance only when administered centrally into the third cerebral ventricle, not peripherally, indicating local tissue rather than circulating vitamin D is functional [27]. Inspired by this, we focused on vitamin D action within specific tissues. A recent study identified 237 VDR target genes via human cells ChIP-seq data [8]. Using eQTLs of these genes as proxies for tissue-specific VDR activation, we found no causal association with BRCA, PCa, HCC or melanoma. Tissue-specific SMR in sarcoma was unfeasible due to heterogeneous origin, limiting verification of the VDBP-sarcoma association.
We comprehensively mined large GWAS and tissue specific eQTL databases to evaluate the causal effect of vitamin D and its tissue-level bioactivity on cancers using MR, complementing prior animal work in intestinal epithelium. Despite broad VDR expressions, we observed no causal effects of local VDR activity on cancers.
Limitations include restriction to European populations, limiting generalizability due to genetic, environment, and baseline vitamin D levels. Non-linear effects of vitamin D and sex-specific effect of prostate cancer and breast cancer were not studied due to data limitation. Vitamin D-gut microbiota interactions, key to anti-tumor effects, were not considered, potentially explaining null findings; future MR studies should integrate microbiome data. We did not incorporate multivariable MR (MVMR) adjusting for lifestyle and metabolic confounders (e.g., BMI, diet, sun exposure proxies) in the current study. Colocalization analyses were not implemented in the current study, primarily because our core SMR results indicate no causal association between the exposure and the outcome. Only 5 types of cancers were analyzed, limiting extrapolation. Limited sample size and eQTLs numbers prevented testing all 237 VDR genes. HCC and sarcoma GWAS had smaller samples, leading to inconsistent power.
In conclusion, our findings indicate no causal relationship between genetically predicted serum 25OHD, VDBP or VDR activity and melanoma, sarcoma, HCC, breast, or prostate cancer risk. While broad vitamin D supplementation to reduce cancer risk is unsupported, our results emphasize the need for targeted strategies and further research into tissue-specific mechanisms, subgroup effects, and microbiome interactions. Future MR analysis may guide the design of large-scale RCTs, advancing personalized and cost-effective cancer prevention.