Introduction
In late December 2019, an outbreak of unexplained cases of pneumonia was reported in Wuhan, China [1]. After determining the aetiological pathogen as a novel Betacoronavirus and clinical presentation of infection similar to Severe Acute Respiratory Syndrome (SARS), the virus has been officially named SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) [2]. The rapid spread of SARS-CoV-2 reported globally in the preceding weeks led to the coronavirus disease 2019 (COVID-19) being declared a public health emergency of international concern [3]. During February 2020 Europe witnessed a massive outbreak in Italy starting in the Lombardy region. Over the following weeks, rapid pandemic spread was observed, at first in the northern, more polluted provinces [4], reaching eventually a total of 97,869 confirmed cases and 10,779 deaths by the end of March in the whole of Italy [5]. The shift of focus towards critically ill individuals with SARS-CoV-2, presenting commonly as complex cases with comorbidities [6], led to a dramatic shortage of access to urgent urological care [7] as well as a huge drop in planned urological surgeries [8]. The disruption in planned surgeries in Italy caused a 35.9% decrease in urooncological surgical activity during four consecutive weeks starting from February 24 [8]. In Paris, the relative reduction of urooncological procedures was estimated at 44% [9]. When considering routine clinical practice, the rapid reorganisation of healthcare led to the increasing prevalence of burnout and physical exhaustion among practising urologists [10]. According to the global survey performed by the UroSoMe group, the COVID-19 outbreak caused a delay of more than 8 weeks in an average of 31% of surgeries, with personnel shortage (27%) being the most common reason for cut-down [11]. To maintain access to the most substantial urological services and to limit clinical harm of delays, the Rapid Reaction Group of the European Association of Urology (EAU) introduced guidelines prioritising diagnosis, surgical treatment, and follow-up during the COVID-19 outbreak [12]. Although EAU guidance adapted previous recommendations to the current situation and delivered crucial support to overloaded healthcare, the timing and extent of the implementation of the guidelines in various regions remained unclear. The absolute necessity of implementing priorities has been successfully contested by Martini Klinik [13], where screening and extensive protective measures facilitated maintaining the volume of radical prostatectomy at pre-pandemic levels without compromising epidemiological safety.
At the time of writing, in Poland, since the first confirmed patient, as reported on March 4, 60,281 COVID-19 cases have been detected, including 1938 COVID-19-related deaths (5) (August 21). The spread of COVID-19 in Poland has been classified early by the World Health Organisation as community transmission, but the national healthcare infrastructure was impacted by the pandemic significantly less than in the majority of Western European countries. Although the Polish National Health Fund recommended postponing elective procedures and a significant number of hospitals were transformed into COVID-19 dedicated centres, a vast majority of caregivers have neither experienced imperative indications of urooncological service disruption nor required prioritising triage of oncological patients.
In this multicentre cross-country study, we aim to determine the impact of the first wave of the COVID-19 pandemic on the surgical treatment of urological cancer patients in Poland.
Material and methods
This nationwide study involved 10 urologic centres in Poland. Data regarding oncological surgeries performed after the outbreak of the first wave of the pandemic in 2020 (March 15, 2020 – May 31, 2020; COVID period) and in the reference period in 2019 (March 15, 2019 – May 31, 2019; pre-COVID period) were retrospectively collected. In all included centres, routine SARS-CoV-2 polymerase chain reaction (PCR) testing was implemented at admission (since April). The analysis included transurethral resection of bladder tumour (TURBT), radical nephrectomy (RN), partial nephrectomy (PN), nephroureterectomy (NU), radical cystectomy (RC), and radical prostatectomy (RP). The number of surgeries performed, preoperative patient characteristics that could facilitate prioritising procedures, and perioperative track in the pre-COVID and COVID period were compared. The total decline in surgical activity was measured by dividing the number of procedures performed in the COVID period by the number of procedures performed in the pre-COVID period. If the number of procedures in the COVID period exceeded the number of procedures performed in the pre-COVID period, the calculation was performed inversely to obtain the rate of increase.
Research involving human participants
All procedures performed during the study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments.
Statistical analysis
Continuous data are shown as mean values supplemented with interquartile ranges (IQR), and categorical data are presented as absolute values and percentages. Data were analysed using non-parametric methods. Differences between median values in compared periods were evaluated using Mann-Whitney U test. Associations between categorical variables were assessed using Fisher’s exact test. For all statistical analyses, a two-sided p-value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.4 software.
Results
Ten urological centres with an availability of 270 beds in 7 out of 16 Polish regions participated in the study (Supplementary Table SI). A total of 1063 and 968 urooncological procedures were reported in the surveyed centres during the pre-COVID and COVID periods, respectively. The most common procedure performed was TURBT (626 surgeries in the pre-COVID period and 500 surgeries in the COVID period), followed by RP (190 and 199 surgeries, respectively), RN (91 and 85 surgeries, respectively), PN (97 and 81 surgeries, respectively), RC (44 and 84 surgeries, respectively), and NU (15 and 19 surgeries, respectively).
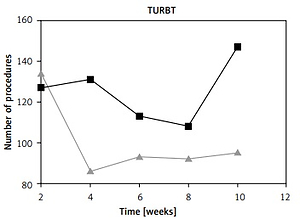
The overall number of urooncological procedures declined by 8.9%. A two-week interval time-trend comparison between both periods regarding the performed procedures is depicted in Figure 1. The highest reduction was observed in TURBT (20.1%) and PN (16.5%). The number of RP and RN remained similar in the COVID period (4.7% and 6.6% change, respectively), whereas the number of RC and NU increased by 90.9% and 26.7%, respectively.
Figure 1
Surgical activity in consecutive weeks* after 15 March, 2020 (COVID) and in the corresponding period in 2019 (pre-COVID). No. – number; TURBT – transurethral resection of bladder tumour; *Time trends are presented for 2-week intervals
Preoperative characteristics and length of hospitalisation (LOH) of evaluated cohorts are presented in Table I (surgeries for urothelial cancer), Table II (surgeries for renal cell cancer), and Table III (radical prostatectomy).
Table I
Characteristics of patients who underwent surgical treatment for urothelial cancer (transurethral resection of bladder tumour, cystectomy, or nephroureterectomy) in pre-COVID and COVID period
[i] ASA – American Society of Anesthesiologists score, Hgb – haemoglobin, HG – high-grade, reTURBT – restaging transurethral resection of bladder tumour, NMIBC – non-muscle-invasive bladder cancer, MIBC - muscle-invasive bladder cancer, cN – clinical nodal staging, cT – clinical local staging, URS – ureterorenoscopy, LOH – length of hospitalisation.
Table II
Characteristics of patients who underwent surgery for renal cell cancer (partial nephrectomy or radical nephrectomy) in pre-COVID and COVID period
Table III
Characteristics of patients who underwent surgical treatment for prostate cancer (radical prostatectomy) in pre-COVID and COVID period
Discussion
This is the first nationwide analysis of urooncological treatment conducted in a country where early, strict lockdown suppressed the SARS-CoV-2 surge during the first weeks of the pandemic. Although subsequent softening of the restrictions resulted in the increasing burden of COVID-19 cases, Poland avoided overloading of the healthcare system during the first wave of the pandemic.
The evolution of a pandemic in Poland during the first months of the COVID-19 crisis differed substantially from its dramatic spread in Spain (386,054 cases – August 21), France (271,905 cases – August 21), or Italy (257,065 cases – August 21), where the COVID-19 outbreak forced rapid reorganisation of national health systems [5]. By August 21 a total of 60,281 COVID-19 cases had been detected in Poland, with 1938 related fatalities [5]. Although the Polish government introduced a national lockdown, the shift of personnel and resources in Poland remained limited, and the decline in crucial specialist medical services was less pronounced than in Western Europe. Our multi-institutional collaborative team previously reported a 22.5% decrease in urgent urologic admissions and an 11.9% decrease in urologic emergency visits during the COVID-19 pandemic in Poland [14]. Although bothersome, the decrease in emergency cases in Poland remained considerably lower than reported in Italian and Portuguese studies where observed declines exceeded 50% [7, 15].
In the present study, we report an overall reduction of planned oncological procedures by 8.9%. This modest decline cannot be compared with the healthcare crisis in Italy, where the estimated procedure reduction during the first phase of the pandemic varied from 35.9% (declared in the multicentre survey) [8] to 67% (Department of Urology in Bergamo Hospital) [16]. Although reports from Italy can be interpreted as being confounded by the particular harm this country initially experienced, the global survey conducted by the UroSoMe Working Group confirmed that Italian observations are relevant worldwide [11]. Responses of 1004 survey participants (mostly Asia, Europe, North America, and South America) revealed 20–53% cut-downs on urooncological surgeries depending on the cancer type. The recent EAU survey showed that 82% of European referral centres declared themsleves to be “much” or “very much” affected by COVID-19 pandemic during the first wave (March 2020), reporting 53%, 41%, 53%, and 52% drops in radical prostatectomies, radical cystectomies, radical/partial nephrectomies, and nephroureterectomies, respectively [17]. Polish observations revealed a different scenario. After stratifying our cohort by procedure, a significant reduction in surgical activity was confirmed only for TURBT (20.1% decline) and PN (16.5% decline), with other major procedures being performed without significant decline (RN, RP), or even with an increase (NU, RC).
The substantial decrease in TURBT might be attributed to the limited inflow of patients from outpatients due to disrupted cystoscopy follow-up as well as postponed haematuria investigations. Given the huge drop in outpatient cystoscopy (77%) after the COVID-19 outbreak revealed in the UroSoMe survey [11], the detection of primary and recurrent urothelial cancer was likely to be altered also in Poland. Considering inpatients, on the other hand, a drop in haematuria cases presenting as urological emergencies could at that moment be as high as 25% [14].
When compared to the 35% mean decrease in surgeries for RCC declared by responders of the UroSoMe survey [11] or the 53% decline reported in the recent EAU survey [17], the 11.7% reduction observed in our study cannot be considered more than moderate. The relative reduction of PN might be attributed to the higher number of lowest-risk kidney tumours being postponed from surgery or proposed active surveillance, as has been suggested by the rapid reaction EAU working group [12, 18].
In Italy, RP and radiotherapy were estimated to decline by 63.6% and 84.6%, respectively, until the end of March [19]. In Poland the volume of RP performed after the COVID-19 outbreak did not decrease. We also failed to validate the UroSoMe survey outcomes, which revealed mean global cut-down on radical prostatectomy exceeding 50% [11]. Given that prostate cancer (PC) is the most deferrable among urological cancers, the question on the risk-benefit ratio of RP during the COVID-19 crisis seems valid as never before. According to the EAU recommendations for the COVID-19 pandemic [12, 18], most prostatectomies for organ-confined intermediate – and high-risk PC can be deferred for 3–6 months without harm, whereas patients with low-risk should be offered active surveillance. The high proportion of patients with grade group (GG) I in biopsy (overall 41.3%) in our study suggests a significant burden of overtreatment in Poland, which is however observed constitutively in Polish series [20, 21]. Nevertheless, RP was the only oncological procedure that presented differences between corresponding periods that can be attributed to oncological triage. Prostatectomy in the COVID period was significantly more likely to be performed in patients with an abnormal digital rectal examination (DRE) (61.6% vs. 47.4%) and/or GG ≥ II patients (63.4% vs. 54%). Of note, abnormal DRE has been recently proposed by the Rapid Reaction Group as one of the major triggers to drive decisions on performing biopsy without delay [12] and might contribute to higher biopsy grading, which constitutes another prioritising factor. Although a trend towards prioritising high-risk PC patients can be noticed, oncological triage in Polish centres presents some similarities with the Martini Klinik, where the implementation of precautions facilitated maintaining PC care at baseline level without compromising epidemic safety [13]. Finally, the COVID-19 outbreak brought a remarkable change in utilising a laparoscopic approach in RP (74.9% vs. 83.2% in COVID and pre-COVID, respectively), which possibly reflects previous concerns about an increased risk of aerosolisation during desufflation [22, 23].
Surprisingly, NU and RC were performed more frequently in the COVID period than in 2019. For nephroureterectomy remaining uncommon procedure mild increase in the number of surgeries suggests rather maintaining the baseline activity. The noted increase in radical cystectomies (90.9%) is a remarkable observation. Given that more than a quarter of muscle-invasive bladder cancer (MIBC) patients fail to be treated within 12 weeks in Poland [24], cystectomy timing seems to contextualise this phenomenon. A considerable load of RC contributes to prolonging oncological waiting lists, whereas this surgery has been recently identified with the highest (36.2%) burden of high-priority patients [25]. Because the prevalence of the pT1 category in Polish patients presenting with primary non-muscle-invasive bladder cancer (NMIBC) is more common than in the available series (49.2% vs. 39%), this burden can be even higher in Poland [26, 27]. We speculate that the Polish National Health Fund recommendation on postponing elective procedures except for oncological treatment might have unloaded waiting lists of RC candidates. Although not statistically significant, the presence of hydronephrosis (39.3% in the COVID period vs. 22.7% in the pre-COVID period) and lesion diameter (mean 40.7 mm vs. 33.2 mm) could have been among the main triggers for surgery during the pandemic.
Despite recent advancements in COVID-19 management and vaccine development, still no clear results and future perspectives can be drawn [28]. European urology is forecasted to face a significant workload requiring maintaining prioritising strategies [17, 29]. Epidemic models suggest that regarding the number of casualties and shorter duration of the lockdown, the most efficient approach would be an initially intensive but further adaptive lockdown strategy [30]. According to this model, mortality would increase linearly with time, even after the first year, but would not overtake the mortality of different models (like continuous lockdown or intermittent lockdown) for a long time. In Poland, the initial “hard” lockdown during the first wave allowed for smooth, gradual inflow of COVID-19 patients without significant influence on urooncological services. Nevertheless, the second wave of the pandemic, which is currently sweeping through Europe, is predicted to have a substantially greater impact on urooncology.
In conclusion, in this study, we report the limited impact of the first wave of the COVID-19 pandemic on urooncological care in Poland. Deployment of staff and limited access to resources during the COVID period have not affected the proceeding of surgical treatment in patients with urological cancers. Simultaneously, it seems that during the initial pandemic period, oncological triage has not been required in the majority of centres, or its impact is yet to be observed. At the moment of publishing data from the first wave of the pandemic, the epidemiological situation is evolving rapidly. Because the second wave of the pandemic in Poland has already presented a dramatically different course, alteration of urooncological service seems unavoidable.
The limitations of our study can be attributed to its retrospective design and the limited period of time that was analysed. Observation times might be too short to determine whether recommendations for urologists during the COVID-19 pandemic have been applied and to what extent. The two periods compared are separated by 1 year, which was chosen to avoid particular confounders (season influence), but it could be a source of other ones (time-dependent changes in surgical services).