Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
PEDIATRICS / SYSTEMATIC REVIEW/META-ANALYSIS
Oral immunotherapy prevents ventilator-associated pneumonia in premature infants: a meta-analysis and systematic review
1
Children's Hospital of Nanjing Medical University, China
Submission date: 2022-07-26
Final revision date: 2022-08-18
Acceptance date: 2022-09-02
Online publication date: 2022-10-03
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Ventilator-associated pneumonia (VAP) prevention and care is essential to the prognosis of premature infants. We aimed to evaluate the effects and safety of oral immune therapy (OIT) in premature infants, to provide evidence for the clinical treatment and nursing care of premature infants.
Material and methods:
We systematically searched PubMed, Embase, Cochrane Library, Web of Science, Cumulative Index of Nursing and Allied Health Literature (CINAHL), China National Knowledge Infrastructure (CNKI), China Biomedical Documentation Service (CBM), Wanfang databases for randomized controlled trials (RCTs) on the effects and safety of OIT in preterm infants until July 16, 2022. Two researchers independently screened the literature and extracted data. Revman 5.3 software was used for data meta-analysis.
Results:
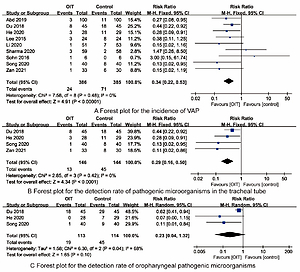
10 RCTs involving 852 premature infants were included, 427 premature infants received OIT. Synthesized outcomes showed that OIT reduced the incidence of VAP [RR=0.34, 95%CI (0.22-0.53)], the detection rate of tracheal tube-causing microorganisms [RR=0.29, 95%CI (0.16-0.50)] and length of hospital stay [MD=-6.60, 95%CI (-11.66, -1.53)] in premature infants (all P<0.05). There were no statistically differences in the detection rate of oropharyngeal pathogenic microorganisms [RR=0.23, 95%CI (0.04-1.32)], duration of mechanical ventilation [MD=-0.67, 95%CI (-1.37, 0.03)], mortality [RR=0.60, 95%CI (0.31, 1.14)] between OIT and control group (all P>0.05).
Conclusions:
OIT is a simple and effective nursing method, which provides a new approach for the prevention of VAP in premature infants. RCTs with high quality, larger sample size and multi-centers are still needed for further verification on the role of OIT in the future.
Ventilator-associated pneumonia (VAP) prevention and care is essential to the prognosis of premature infants. We aimed to evaluate the effects and safety of oral immune therapy (OIT) in premature infants, to provide evidence for the clinical treatment and nursing care of premature infants.
Material and methods:
We systematically searched PubMed, Embase, Cochrane Library, Web of Science, Cumulative Index of Nursing and Allied Health Literature (CINAHL), China National Knowledge Infrastructure (CNKI), China Biomedical Documentation Service (CBM), Wanfang databases for randomized controlled trials (RCTs) on the effects and safety of OIT in preterm infants until July 16, 2022. Two researchers independently screened the literature and extracted data. Revman 5.3 software was used for data meta-analysis.
Results:
10 RCTs involving 852 premature infants were included, 427 premature infants received OIT. Synthesized outcomes showed that OIT reduced the incidence of VAP [RR=0.34, 95%CI (0.22-0.53)], the detection rate of tracheal tube-causing microorganisms [RR=0.29, 95%CI (0.16-0.50)] and length of hospital stay [MD=-6.60, 95%CI (-11.66, -1.53)] in premature infants (all P<0.05). There were no statistically differences in the detection rate of oropharyngeal pathogenic microorganisms [RR=0.23, 95%CI (0.04-1.32)], duration of mechanical ventilation [MD=-0.67, 95%CI (-1.37, 0.03)], mortality [RR=0.60, 95%CI (0.31, 1.14)] between OIT and control group (all P>0.05).
Conclusions:
OIT is a simple and effective nursing method, which provides a new approach for the prevention of VAP in premature infants. RCTs with high quality, larger sample size and multi-centers are still needed for further verification on the role of OIT in the future.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.