Introduction
Pericoronitis refers to inflammation of soft tissue around the crown of the third molar. It often occurs in 18–25-year-old people, the lower jaw is more common, and there are acute and chronic differences [1, 2]. The form of acute inflammation is more common in clinical practice. In the initial stage of inflammation, the patients only felt slight pain and discomfort in the affected area, and the pain increased during chewing, swallowing and mouth opening activities; the inflammation continued to worsen; local spontaneous shooting pain occurred and radiated to the ipsilateral head and face, resulting in varying degrees of limitation of opening the mouth [3–5]. Timely treatment and nursing of pericoronitis is of great significance for the quality of life of patients with pericoronitis [6].
The treatment of pericoronitis is mainly by enhancing the resistance of patients, controlling infection and promoting the dissipation of inflammation [7]. At the same time, surgical treatment of the diseased teeth should be carried out to control the disease and prevent recurrence. Treatment methods include systemic treatment, local treatment, and treatment of affected teeth [8]. Systemic treatment is mainly oral antibacterial, heat-clearing and detoxification and other drugs. Local treatment is mainly for doctors to clean around the source teeth and point to other drugs that can relieve symptoms to achieve the purpose of treatment. The treatment of the affected teeth involves treating the diseased teeth after the acute inflammation has subsided, including tooth position, pericoronal gingival flap wedge resection or tooth extraction [9]. Ornidazole is a new generation of anti-anaerobic drugs [10]. In recent years, there have been many clinical reports of ornidazole in the treatment of pericoronitis of wisdom teeth, but the results are not consistent. In order to further objectively evaluate the clinical efficacy of ornidazole for pericoronitis, this study aimed to evaluate the effect of ornidazole on pericoronitis by meta-analysis, to provide evidence-based medical evidence for the treatment of pericoronitis in clinical practice.
Material and methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [11]. Ethical approval was not necessary since this study was a meta-analysis and systematic review.
The two researchers independently searched the PubMed, Clinical trials, EMBASE, Science Direct, Cochrane Library, China National Knowledge Infrastructure (CNKI), Weipu and Wanfang databases to find randomized controlled trials (RCTs) of ornidazole in the treatment of pericoronitis from the establishment of the database to March 15, 2023. The search terms used in this meta-analysis were as follows: (“ornidazole” OR “Pusili”) AND (“pericoronitis” OR “third molar” OR “wisdom teeth”). All searches adopted the method of combining subject words with free words in every database search.
The RCT inclusion criteria of this meta-analysis were as follows: (1) study type: all RCT patients with pericoronitis treated with iodoglycerin or ornidazole, the language was limited to Chinese and English; (2) population: patients ≥ 18 years old, clinically diagnosed with wisdom tooth pericoronitis; (3) intervention: both the test group and the control group received routine treatment with iodoglycerin, and the patients in the test group were treated with ornidazole on the basis of the treatment of the control group; (4) outcome indicators: RCT reported the corresponding treatment indicators, such as effective rate, oral bacterial density, and pain disappearance time. The exclusion criteria of this meta-analysis were as follows: repeated reports, reviews, conference papers, literature with incomplete original data.
The included literature items were screened by two researchers independently according to the inclusion and exclusion criteria, and then the relevant data were extracted for meta-analysis. The data extracted by this meta-analysis included: general data included in the study (title, first author’s name, publication time, literature source); characteristics included in RCT (general situation of study subjects, intervention measures, random methods, blind setting); outcome indicators: If there were differences, they could be resolved by the discussion of two researchers or decided by a third researcher.
The methodological quality of included RCTs was evaluated according to the bias risk assessment criteria in the Cochrane evaluator’s manual. The evaluation contents included: the specific method of random sequence generation; whether to use matching hiding; whether to use a blind method; whether the result data were complete; whether there was selective reporting of results; whether there were other biases. Each item was rated as “yes”, “no” or “unclear”, where “yes” indicated low bias, “no” indicated high bias, and “unclear” indicated lack of relevant information or uncertainty of bias.
Statistical analysis
This study used Review Manager 5.3 software for statistical analysis. The binary risk ratio (RR) was calculated as the effect statistic for the counting data, and the mean difference (MD) was calculated as the effect statistic for the measurement data; the effect statistics were expressed as the 95% confidence interval (95% CI). According to the possible heterogeneity factors, subgroup analysis and the χ2 test were used to test the heterogeneity of the included study. P > 0.05, I2 < 50% indicated that the homogeneity among the research results was good, so the fixed effect model was used for meta-analysis; otherwise, the random effect model was used when p < 0.05, I2 ≥ 50%. The results of meta-analysis were expressed by forest plots, and the publication bias was analyzed by the funnel plot. The test level of this meta-analysis was α = 0.05.
Results
In this meta-analysis, 177 literature items were identified in the initial searches. After screening one by one, 16 RCTs were finally included. The literature screening process is shown in Figure 1.
The characteristics of included RCTs are shown in Table I. A total of 2004 patients with pericoronitis were involved in 16 RCTs [12–27], including 1038 cases in the ornidazole group and 966 cases in the control group. Most included RCTs compared the use of ornidazole versus iodoglycerin, and the most common ornidazole dose was 0.25 g/time, once/day for 5 days.
Table I
Characteristics of included RCTs
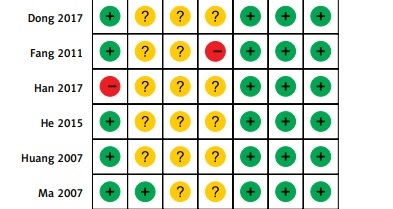
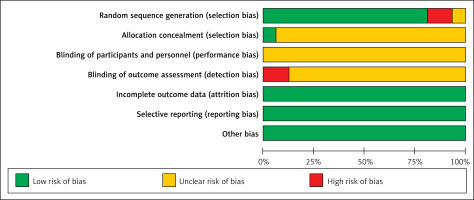
As shown in Figures 2 and 3, all included studies mentioned that the randomization was used in their studies, one RCT [19] did not report the methods for random sequence generation, while two RCTs [13, 23] reported that wrong methods of random sequence generation were used and were rated as high risk of bias. Most included RCTs did not report the blind study design. All the RCTs provided the necessary data and outcomes for selective reporting and other bias evaluations.
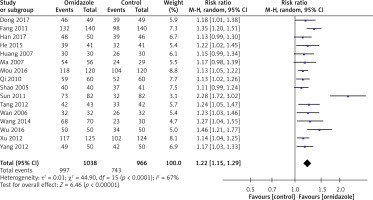
As shown in Figure 4, all 16 RCTs reported the effective rate of treatment. After the heterogeneity test (p < 0.001, I2 = 67%), there was obvious heterogeneity among the studies, so the random effect model was used for analysis. The results of meta-analysis showed that the effective rate of ornidazole treatment was significantly higher than that of the routine treatment group (RR = 1.22, 95% CI (1.15, 1.29), p < 0.001).
As shown in Table II, the results of meta-analysis showed that ornidazole treatment was beneficial to reduce the oral bacterial density (MD = –26.13, 95% CI (−32.08, –21.51)), time to pain disappearance (MD = –0.64, 95% CI (−0.92, –0.17)) and time to disappearance of redness and swelling of teeth crown (MD = –1.45, 95% CI (−2.43, –1.01)) compared to the routine treatment (all p < 0.05).
Table II
Meta-analysis on oral bacterial density, time to pain disappearance, time to disappearance of redness and swelling of teeth crown
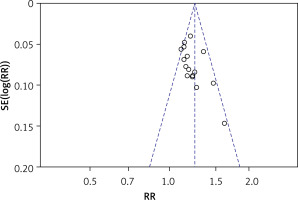
Sensitivity analyses, which analyze the influence of one study on the overall risk estimate by removing one RCT in turn, indicated that the overall risk estimates were not substantially changed by any single study. The funnel chart was drawn with RR as the horizontal coordinate and log (RR) as the ordinate. Publication bias analysis of the efficiency in the treatment period was carried out. As shown in Figure 5, each point was basically symmetrical on both sides of the baseline. The Egger test result did not indicate any publication bias (p = 0.206).
Discussion
The results of this meta-analysis showed that ornidazole significantly improves the clinical efficacy, and reduces the duration of pain and pericoronal redness and swelling, which may merit clinical application for the treatment of pericoronitis. Ornidazole is a new type of third-generation nitroimidazole anti-anaerobic drug, which has the advantages of strong antibacterial activity, good tolerance, short course of treatment and less adverse reactions [28]. Ornidazole is a third generation nitroimidazole; its minimum inhibitory concentration and minimum bactericidal concentration are lower than metronidazole and tinidazole, and its antibacterial advantage is obvious [29, 30]. At the same time, the half-life of plasma elimination is 14.4 h, which is longer than that of metronidazole and tinidazole, so the efficacy lasts longer [31].
The cause of pericoronitis is that the eruption of the third molar is limited, resulting in part or even all of the crown being surrounded by gingival tissue, forming a blind bag, which provides favorable conditions for the growth and reproduction of anaerobic bacteria, resulting in inflammation, redness and swelling of the surrounding soft tissue [32, 33]. Early and effective treatment of pericoronitis is very important for the prognosis of patients. Compared with conventional therapy in the treatment of pericoronitis, ornidazole combined with conventional therapy can make the local drug concentration high, with a relatively long-lasting effect, a quick effect and less adverse reactions [34, 35]. It has been shown that ornidazole can kill the anaerobes that cause gingival inflammation, with a killing rate of 90.5% [36]. Pericoronitis is generally treated locally by rinsing the pericoronal blind bag with 3% hydrogen peroxide and normal saline, and then covered with iodoglycerin or other drugs, which has a certain curative effect but cannot kill the bacteria in the pericoronal bag [37]. The total effective rate of ornidazole in the treatment of pericoronitis is significantly higher. The disappearance time of pain, pericoronal redness and swelling and oral bacterial density after treatment with ornidazole have significantly lower values than in patients treated with other therapy. There are a few reports that individual patients have mild adverse reactions, such as nausea, loss of appetite, dizziness, headache, and so on [38]. We can further consider the combination of drugs to make up for the shortcomings of clinical use of ornidazole.
There are many shortcomings of this meta-analysis that deserve consideration. Firstly, the quality of the included RCTs is uneven, the overall sample size is small, and there are some deficiencies in the blind method of clinical research, which raises some doubts as to the degree of objectivity of the research results. Secondly, all the studies included in the meta-analysis have been carried out on Asian subjects; it is unclear whether the drug will exert the same effect on other ethnicities. Thirdly, due to the limitations of the included data, we are unable to do further subgroup analysis of the course of treatment, gender and other factors. More randomized double-blind controlled trials with large samples in different ethnicities and populations are needed to provide more convincing evidence.
In conclusion, the results of the meta-analysis showed that ornidazole combined with routine therapy is helpful to improve the clinical efficacy, reduce the duration of pain and reduce the density of oral bacteria. The promotion of ornidazole combined with conventional therapy in the treatment of wisdom tooth pericoronitis still needs the support of large clinical sample, multicenter RCTs with higher quality and larger sample sizes to provide more reliable evidence for the treatment of pericoronitis.