Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
IMMUNOLOGY / RESEARCH PAPER
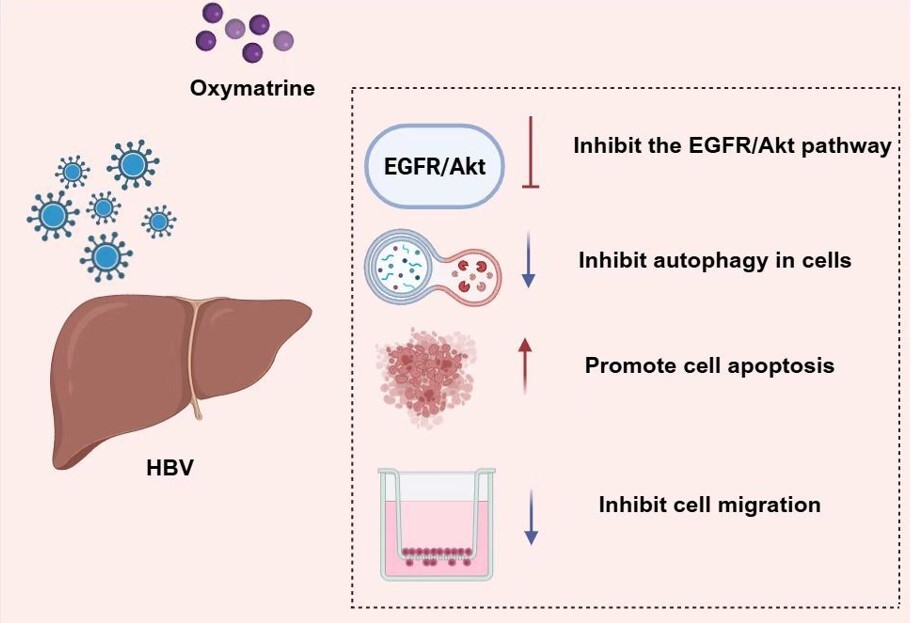
Oxymatrine blocked mother-to-child transmission of hepatitis B virus by modulating autophagy in trophoblast cells
1
Zhejiang Provincial People’s Hospital, China
2
Zhejiang Chinese Medical University, China
These authors had equal contribution to this work
Submission date: 2025-01-24
Final revision date: 2025-05-24
Acceptance date: 2025-07-12
Online publication date: 2025-08-23
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Mother-to-child transmission is a significant pathway for chronic carriers of the hepatitis B virus (HBV) in China. In this study, we aimed to investigate the role and mechanism of Oxymatrine (OMT) in preventing mother-to-child transmission of HBV.
Material and methods:
To simulate MTCT, we utilized the HBV-infected human trophoblast cell line HTR-8/SVneo, which serves as a relevant model for studying HBV transmission at the maternal-fetal interface. The replication capacity of HBV in these cells was quantified using enzyme-linked immunosorbent assay (ELISA) and real-time fluorescence polymerase chain reaction (PCR). The expression levels of key autophagy markers were assessed using Western blotting, providing insights into the autophagy-related mechanisms potentially involved. Additionally, the Cell Counting Kit-8 (CCK-8) assay was employed to measure the proliferation of trophoblast cells under different treatment conditions.
Results:
We found that OMT inhibited HBV DNA replication in HBV-infected trophoblast cells. Additionally, OMT suppressed the proliferation and autophagy in HBV-infected trophoblast cells. This suggested that OMT might effectively block mother-to-child transmission of HBV. Mechanistically, OMT appears to prevent mother-to-child transmission of HBV by inhibiting the EGFR/Akt pathway.
Conclusions:
OMT inhibited HBV transmission by regulating the EGFR/Akt pathway, and this study may provide new ideas and methods for the prevention of mother-to-child transmission of HBV infection during pregnancy.
Mother-to-child transmission is a significant pathway for chronic carriers of the hepatitis B virus (HBV) in China. In this study, we aimed to investigate the role and mechanism of Oxymatrine (OMT) in preventing mother-to-child transmission of HBV.
Material and methods:
To simulate MTCT, we utilized the HBV-infected human trophoblast cell line HTR-8/SVneo, which serves as a relevant model for studying HBV transmission at the maternal-fetal interface. The replication capacity of HBV in these cells was quantified using enzyme-linked immunosorbent assay (ELISA) and real-time fluorescence polymerase chain reaction (PCR). The expression levels of key autophagy markers were assessed using Western blotting, providing insights into the autophagy-related mechanisms potentially involved. Additionally, the Cell Counting Kit-8 (CCK-8) assay was employed to measure the proliferation of trophoblast cells under different treatment conditions.
Results:
We found that OMT inhibited HBV DNA replication in HBV-infected trophoblast cells. Additionally, OMT suppressed the proliferation and autophagy in HBV-infected trophoblast cells. This suggested that OMT might effectively block mother-to-child transmission of HBV. Mechanistically, OMT appears to prevent mother-to-child transmission of HBV by inhibiting the EGFR/Akt pathway.
Conclusions:
OMT inhibited HBV transmission by regulating the EGFR/Akt pathway, and this study may provide new ideas and methods for the prevention of mother-to-child transmission of HBV infection during pregnancy.
REFERENCES (42)
2.
Chen, Y. and Z. Tian, HBV-Induced Immune Imbalance in the Development of HCC. Front Immunol, 2019. 10: p. 2048.
3.
Jing, W., J. Liu, and M. Liu, Eliminating mother-to-child transmission of HBV: progress and challenges in China. Front Med, 2020. 14(1): p. 21-29.
4.
Chen, Y., et al., Role of maternal viremia and placental infection in hepatitis B virus intrauterine transmission. Microbes Infect, 2013. 15(5): p. 409-15.
5.
Delorme-Axford, E., et al., Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci U S A, 2013. 110(29): p. 12048-53.
6.
Liu, S., et al., Autophagy: Regulator of cell death. Cell Death Dis, 2023. 14(10): p. 648.
7.
Chiramel, A.I. and S.M. Best, Role of autophagy in Zika virus infection and pathogenesis. Virus Res, 2018. 254: p. 34-40.
8.
Miller, K., et al., Coronavirus interactions with the cellular autophagy machinery. Autophagy, 2020. 16(12): p. 2131-2139.
9.
Chan, S.T. and J.J. Ou, Hepatitis C Virus-Induced Autophagy and Host Innate Immune Response. Viruses, 2017. 9(8).
10.
Gao, H., et al., The Role of Autophagy in the Mother-to-Child Transmission of Pregnant Women With a High Level of HBV DNA. Front Cell Infect Microbiol, 2022. 12: p. 850747.
11.
Cui, H., et al., Hepatitis B virus X protein modifies invasion, proliferation and the inflammatory response in an HTR-8/SVneo cell model. Oncol Rep, 2015. 34(4): p. 2090-8.
12.
Dai, Y.J., et al., Recent advances of traditional Chinese medicine on the prevention and treatment of COVID-19. Chin J Nat Med, 2020. 18(12): p. 881-889.
13.
Li, T. and T. Peng, Traditional Chinese herbal medicine as a source of molecules with antiviral activity. Antiviral Res, 2013. 97(1): p. 1-9.
14.
Ren, W., et al., Research progress of traditional Chinese medicine against COVID-19. Biomed Pharmacother, 2021. 137: p. 111310.
15.
Lan, X., et al., Oxymatrine exerts organ- and tissue-protective effects by regulating inflammation, oxidative stress, apoptosis, and fibrosis: From bench to bedside. Pharmacol Res, 2020. 151: p. 104541.
16.
Sang, X., et al., T cell--associated immunoregulation and antiviral effect of oxymatrine in hydrodynamic injection HBV mouse model. Acta Pharm Sin B, 2017. 7(3): p. 311-318.
17.
Xu, W.S., et al., Effect of oxymatrine on the replication cycle of hepatitis B virus in vitro. World J Gastroenterol, 2010. 16(16): p. 2028-37.
18.
Song, W.J., et al., Oral oxymatrine preparation for chronic hepatitis B: A systematic review of randomized controlled trials. Chin J Integr Med, 2016. 22(2): p. 141-9.
19.
Cui, H., J. Chen, and Q. Na, Effect of hepatitis B virus infection on trophoblast cell line (HTR-8/SVneo) and choriocarcinoma cell line (JEG3) is linked to CD133-2 (AC141) expression. Am J Transl Res, 2016. 8(7): p. 3235-40.
20.
Cheung, K.W., M.T.Y. Seto, and T.T. Lao, Prevention of perinatal hepatitis B virus transmission. Arch Gynecol Obstet, 2019. 300(2): p. 251-259.
21.
Ma, L., et al., Mother-to-child transmission of HBV: review of current clinical management and prevention strategies. Rev Med Virol, 2014. 24(6): p. 396-406.
22.
Mavilia, M.G. and G.Y. Wu, Mechanisms and Prevention of Vertical Transmission in Chronic Viral Hepatitis. J Clin Transl Hepatol, 2017. 5(2): p. 119-129.
23.
Bai, G., et al., The study on the role of hepatitis B virus X protein and apoptosis in HBV intrauterine infection. Arch Gynecol Obstet, 2012. 285(4): p. 943-9.
24.
Abbas, Y., et al., Investigation of human trophoblast invasion in vitro. Hum Reprod Update, 2020. 26(4): p. 501-513.
25.
Lai, T.H., H.T. Chen, and W.B. Wu, Trophoblast coculture induces intercellular adhesion molecule-1 expression in uterine endometrial epithelial cells through TNF-α production: Implication of role of FSH and ICAM-1 during embryo implantation. J Reprod Immunol, 2022. 152: p. 103650.
26.
Jackson, W.T., Autophagy as a broad antiviral at the placental interface. Autophagy, 2013. 9(12): p. 1905-7.
27.
Liu, D.X., et al., Exosomes derived from HBV-associated liver cancer promote chemoresistance by upregulating chaperone-mediated autophagy. Oncol Lett, 2019. 17(1): p. 323-331.
28.
He, Q., et al., Dexamethasone Stimulates Hepatitis B Virus (HBV) Replication Through Autophagy. Med Sci Monit, 2018. 24: p. 4617-4624.
29.
Wu, S.Y., S.H. Lan, and H.S. Liu, Autophagy and microRNA in hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol, 2016. 22(1): p. 176-87.
30.
Wang, D.D., et al., Relationship between Maternal PBMC HBV cccDNA and HBV Serological Markers and its Effect on HBV Intrauterine Transmission. Biomed Environ Sci, 2019. 32(5): p. 315-323.
31.
Huan, D.Q., N.Q. Hop, and N.T. Son, Oxymatrine: A current overview of its health benefits. Fitoterapia, 2023. 168: p. 105565.
32.
Lin, M., et al., Inhibition of the replication of hepatitis B virus in vitro by oxymatrine. J Int Med Res, 2009. 37(5): p. 1411-9.
33.
Lu, L.G., et al., Inhibitory effect of oxymatrine on serum hepatitis B virus DNA in HBV transgenic mice. World J Gastroenterol, 2004. 10(8): p. 1176-9.
34.
Jiang, X., et al., Oral oxymatrine for hepatitis B cirrhosis: A systematic review protocol. Medicine (Baltimore), 2018. 97(49): p. e13482.
35.
Lu, L.G., et al., Oxymatrine therapy for chronic hepatitis B: a randomized double-blind and placebo-controlled multi-center trial. World J Gastroenterol, 2003. 9(11): p. 2480-3.
36.
Cui, X., et al., Blockage of EGFR/AKT and mevalonate pathways synergize the antitumor effect of temozolomide by reprogramming energy metabolism in glioblastoma. Cancer Commun (Lond), 2023. 43(12): p. 1326-1353.
37.
Jing, X., et al., IGF2BP3-EGFR-AKT axis promotes breast cancer MDA-MB-231 cell growth. Biochim Biophys Acta Mol Cell Res, 2023. 1870(8): p. 119542.
38.
Wang, W., et al., HBxAg suppresses cell apoptosis and promotes the secretion of placental hormones in human placental trophoblasts via activation of the EGFR/Akt pathway. Cell Biol Int, 2018. 42(2): p. 237-247.
39.
Lin, Y., et al., HBxAg promotes HBV replication and EGFR activation in human placental trophoblasts. Exp Ther Med, 2021. 22(5): p. 1211.
40.
Guo, B., et al., Oxymatrine targets EGFR(p-Tyr845) and inhibits EGFR-related signaling pathways to suppress the proliferation and invasion of gastric cancer cells. Cancer Chemother Pharmacol, 2015. 75(2): p. 353-63.
41.
Halim, C.E., et al., Anti-cancer effects of oxymatrine are mediated through multiple molecular mechanism(s) in tumor models. Pharmacol Res, 2019. 147: p. 104327.
42.
Dai, Z., et al., Oxymatrine induces cell cycle arrest and apoptosis and suppresses the invasion of human glioblastoma cells through the EGFR/PI3K/Akt/mTOR signaling pathway and STAT3. Oncol Rep, 2018. 40(2): p. 867-876.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.