Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
CLINICAL RESEARCH
Pyruvate kinase M2 plays a pivotal role in promoting PD-L1 expression and malignant behaviors of hepatoma cells
1
Key Laboratory of Tropical Translational Medicine, Ministry of Education, and Hainan Provincial Key Laboratory of Carcinogenesis and Intervention, Hainan Medical University, Haikou, Hainan Province, China
2
Department of Medical Oncology, Second Affiliated Hospital, Hainan Medical University, Haikou, Hainan Province, China
These authors had equal contribution to this work
Submission date: 2025-01-21
Final revision date: 2025-07-09
Acceptance date: 2025-07-14
Online publication date: 2025-08-23
Corresponding author
Mingyue Zhu
Key Laboratory of Tropical Translational Medicine Ministry of Education, and Hainan Provincial Key Laboratory of Carcinogenesis and Intervention Hainan Medical University 3 Xueyuan Road Longhua District Hainan Medical University Haikou 571199 Hainan, China
Key Laboratory of Tropical Translational Medicine Ministry of Education, and Hainan Provincial Key Laboratory of Carcinogenesis and Intervention Hainan Medical University 3 Xueyuan Road Longhua District Hainan Medical University Haikou 571199 Hainan, China
Mengsen Li
Key Laboratory of Tropical Translational Medicine Ministry of Education, and Hainan Provincial Key Laboratory of Carcinogenesis and Intervention Hainan Medical University 3 Xueyuan Road Longhua District Hainan Medical University Haikou 571199 Hainan, China
Key Laboratory of Tropical Translational Medicine Ministry of Education, and Hainan Provincial Key Laboratory of Carcinogenesis and Intervention Hainan Medical University 3 Xueyuan Road Longhua District Hainan Medical University Haikou 571199 Hainan, China
KEYWORDS
pyruvate kinase M2hepatocellular carcinomaWarburg effectPD-L1malignant behaviorsPI3K/Akt signaling pathway
TOPICS
ABSTRACT
Introduction:
Pyruvate kinase M2 (PKM2) is a key rate-limiting enzyme that regulates glucose metabolic reprogramming (Warburg effect), but the correlation between PKM2 and PD-L1 or malignant behaviors in hepatocellular carcinoma (HCC) cells remains unknown. This study explored the role of PKM2 and the Warburg effect on the expression of PD-L1 and the malignant behaviors of HCC cells.
Material and methods:
The relationship between the Warburg effect and key enzymes and signaling pathways was analyzed using bioinformatics; the expression of PD-L1, PKM2, and hexokinase 2 (HK2) in 30 patients’ HCC tissues and paired para-cancerous tissues was detected by immunohistochemistry and Western blotting. Short hairpin RNA (shRNA) silencing the expression of PKM2 was used to explore its influence on the expression of PD-L1 in HCC cells. The malignant behaviors of HCC cells were detected by scratch test, plate cloning experiment, Transwell migration experiment, and EdU staining; the concentrations of lactic acid, pyruvate, and ATP and the consumption of glucose were detected using a reagent kit.
Results:
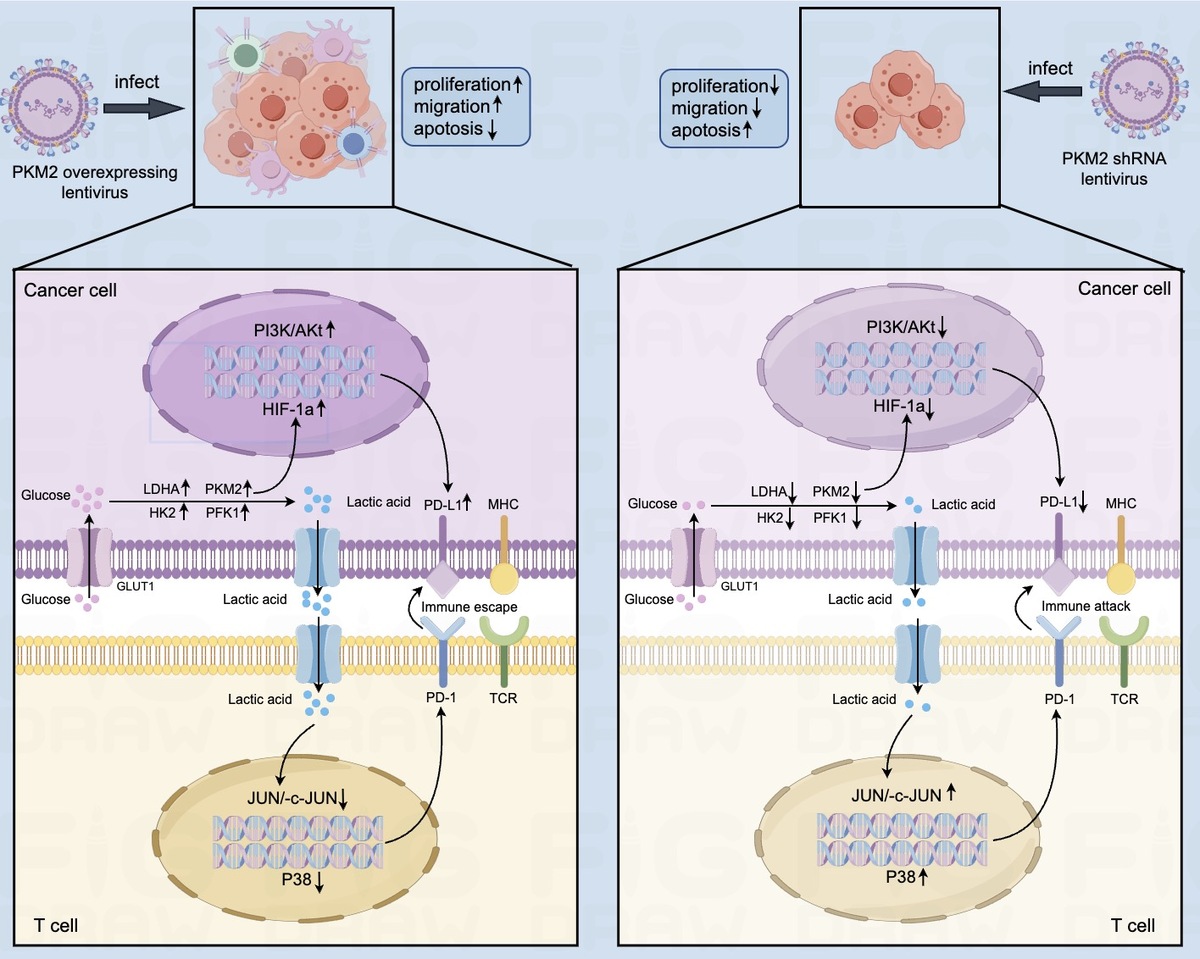
Biological information database and detection of HCC tissues and paired para-cancerous tissues showed that the expression levels of PKM2 and PD-L1 were significantly higher in HCC tissues than in paired para-cancerous tissues, and the expression of PKM2 was positively correlated with PD-L1 expression. PKM2 could promote the proliferation and migration of HCC cells, and stimulate the expression of PD-L1 through activating the PI3K/Akt signaling pathway in HCC cells.
Conclusions:
PKM2 was able to upregulate the expression of PD-L1 and stimulate the malignant behaviors of HCC cells. Targeting PKM2 is a promising strategy for liver cancer treatment.
Pyruvate kinase M2 (PKM2) is a key rate-limiting enzyme that regulates glucose metabolic reprogramming (Warburg effect), but the correlation between PKM2 and PD-L1 or malignant behaviors in hepatocellular carcinoma (HCC) cells remains unknown. This study explored the role of PKM2 and the Warburg effect on the expression of PD-L1 and the malignant behaviors of HCC cells.
Material and methods:
The relationship between the Warburg effect and key enzymes and signaling pathways was analyzed using bioinformatics; the expression of PD-L1, PKM2, and hexokinase 2 (HK2) in 30 patients’ HCC tissues and paired para-cancerous tissues was detected by immunohistochemistry and Western blotting. Short hairpin RNA (shRNA) silencing the expression of PKM2 was used to explore its influence on the expression of PD-L1 in HCC cells. The malignant behaviors of HCC cells were detected by scratch test, plate cloning experiment, Transwell migration experiment, and EdU staining; the concentrations of lactic acid, pyruvate, and ATP and the consumption of glucose were detected using a reagent kit.
Results:
Biological information database and detection of HCC tissues and paired para-cancerous tissues showed that the expression levels of PKM2 and PD-L1 were significantly higher in HCC tissues than in paired para-cancerous tissues, and the expression of PKM2 was positively correlated with PD-L1 expression. PKM2 could promote the proliferation and migration of HCC cells, and stimulate the expression of PD-L1 through activating the PI3K/Akt signaling pathway in HCC cells.
Conclusions:
PKM2 was able to upregulate the expression of PD-L1 and stimulate the malignant behaviors of HCC cells. Targeting PKM2 is a promising strategy for liver cancer treatment.
REFERENCES (64)
1.
Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021; 7: 6.
2.
Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016; 2: 16018.
3.
Ouyang T, Kan X, Zheng C. Immune checkpoint inhibitors for advanced hepatocellular carcinoma: monotherapies and combined therapies. Front Oncol 2022; 12: 898964.
4.
Chen Y, Hu H, Yuan X, Fan X, Zhang C. Advances in immune checkpoint inhibitors for advanced hepatocellular carcinoma. Front Immunol 2022; 13: 896752.
5.
Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 2020; 10: 727-42.
6.
Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms controlling PD-L1 expression in cancer. Mol Cell 2019; 76: 359-70.
7.
Xu F, Jin T, Zhu Y, Dai C. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res 2018; 37: 110.
8.
Tang Q, Chen Y, Li X, et al. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol 2022; 13: 964442..
9.
Xia P, Zhang H, Lu H, et al. METTL5 stabilizes c-Myc by facilitating USP5 translation to reprogram glucose metabolism and promote hepatocellular carcinoma progression. Cancer Commun (Lond) 2023; 43: 338-64.
10.
Zhou Q, Yin Y, Yu M, et al. GTPBP4 promotes hepatocellular carcinoma progression and metastasis via the PKM2 dependent glucose metabolism. Redox Biol 2022; 56: 102458.
11.
Peñuelas-Haro I, Espinosa-Sotelo R, Crosas-Molist E, et al. The NADPH oxidase NOX4 regulates redox and metabolic homeostasis preventing HCC progression. Hepatology 2023; 78: 416-33.
12.
Du D, Liu C, Qin M, et al. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm Sin B 2022; 12: 558-80.
13.
Liberti MV, Locasale JW. The Warburg effect: How does it benefit cancer cells? Trends Biochem Sci 2016; 41: 211-8.
14.
Gatenby RA, Gawlinski ET. A reaction-diffusion model of cancer invasion. Cancer Res 1996; 56: 5745-53.
15.
Estrella V, Chen T, Lloyd M, et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res 2013; 73: 1524-35.
16.
Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 2015; 162: 1229-41.
17.
Wiese EK, Hitosugi T. Tyrosine kinase signaling in cancer metabolism: PKM2 paradox in the Warburg effect. Front Cell Dev Biol 2018; 6: 79.
18.
Luo W, Semenza GL. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol Metab 2012; 23: 560-6.
19.
Ishfaq M, Bashir N, Riaz SK, et al. Expression of HK2, PKM2, and PFKM is associated with metastasis and late disease onset in breast cancer patients. Genes (Basel) 2022; 13: 549.
20.
Azoitei N, Becher A, Steinestel K, et al. PKM2 promotes tumor angiogenesis by regulating HIF-1 through NF-B activation. Mol Cancer 2016; 15: 3.
21.
Wang Y, Hao F, Nan Y, et al. PKM2 inhibitor Shikonin overcomes the cisplatin resistance in bladder cancer by inducing necroptosis. Int J Biol Sci 2018; 14: 1883-91.
22.
Li TE, Wang S, Shen XT, et al. PKM2 drives hepatocellular carcinoma progression by inducing immunosuppressive microenvironment. Front Immunol 2020; 11: 589997.
23.
Zhang Z, Deng X, Liu Y, Liu Y, Sun L, Chen F. PKM2, function and expression and regulation. Cell Biosci 2019; 9: 52.
24.
Yang W, Xia Y, Hawke D, et al. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell 2012; 150: 685-96.
25.
Xu F, Na L, Li Y, Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci 2020; 10: 54.
26.
Tang X, Yang J, Shi A, et al. CD155 cooperates with PD-1/PD-L1 to promote proliferation of esophageal squamous cancer cells via PI3K/Akt and MAPK signaling pathways. Cancers(Basel) 2022; 14: 5610.
27.
Wang F, Yang L, Xiao M, et al. PD-L1 regulates cell proliferation and apoptosis in acute myeloid leukemia by activating PI3K-AKT signaling pathway. Sci Rep 2022; 12: 11444.
28.
Piao W, Li L, Saxena V, et al. PD-L1 signaling selectively regulates T cell lymphatic transendothelial migration. Nat Commun 2022; 13: 2176.
29.
Quan Z, Yang Y, Zheng H, et al. Clinical implications of the interaction between PD-1/PD-L1 and PI3K/AKT/mTOR pathway in progression and treatment of non-small cell lung cancer. J Cancer 2022; 13: 3434-43.
30.
Atefi M, Avramis E, Lassen A, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res 2014; 20: 3446-57.
31.
Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 2007; 13: 84-8.
32.
Gao Y, Yang J, Cai Y, et al. IFN--mediated inhibition of lung cancer correlates with PD-L1 expression and is regulated by PI3K-AKT signaling. Int J Cancer 2018; 143: 931-43.
33.
Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer 2020; 20: 74-88.
34.
Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2020; 17: 139-52.
35.
Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer 2020; 1873: 188314.
36.
Liu CY, Chen KF, Chen PJ. Treatment of liver cancer. Cold Spring Harb Perspect Med 2015; 5: a021535.
37.
Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021; 18: 525-43.
38.
Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009; 15: 971-9.
39.
Pazgan-Simon M, Szymanek-Pasternal A, Górka-Dynysiewicz J, et al. Serum chemerin level in patients with liver cirrhosis and primary and multifocal hepatocellular carcinoma with consideration of insulin level. Arch Med Sci 2024; 20: 1504-10.
40.
Fu Y, Liu S, Zeng S, Shen H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res 2019; 38: 396.
41.
Wen L, Xin B, Wu P, et al. An efficient combination immunotherapy for primary liver cancer by harmonized activation of innate and adaptive immunity in mice. Hepatology 2019; 69: 2518-32.
42.
Yap TA, Parkes EE, Peng W, Moyers JT, Curran MA, Tawbi HA. Development of immunotherapy combination strategies in cancer. Cancer Discov 2021; 11: 1368-97.
43.
Zhu S, Zhang T, Zheng L, et al. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol 2021; 14: 156.
44.
Satriano L, Lewinska M, Rodrigues PM, Banales JM, Andersen JB. Metabolic rearrangements in primary liver cancers: cause and consequences. Nat Rev Gastroenterol Hepatol 2019; 16: 748-66.
45.
Dayton TL, Jacks T, Vander Heiden MG. PKM2, cancer metabolism, and the road ahead. EMBO Rep 2016; 17: 1721-30.
46.
Zhu S, Guo Y, Zhang X, et al. Pyruvate kinase M2 (PKM2) in cancer and cancer therapeutics. Cancer Lett 2021; 503: 240-8.
47.
Madhok BM, Yeluri S, Perry SL, Hughes TA, Jayne DG. Targeting glucose metabolism: an emerging concept for anticancer therapy. Am J Clin Oncol 2011; 34: 628-35.
48.
Ciscato F, Ferrone L, Masgras I, Laquatra C, Rasola A. Hexokinase 2 in cancer: a prima donna playing multiple characters. Int J Mol Sci 2021; 22: 4716.
49.
Jiao L, Zhang HL, Li DD, et al. Regulation of glycolytic metabolism by autophagy in liver cancer involves selective autophagic degradation of HK2(hexokinase 2). Autophagy 2018; 14: 671-84.
50.
Li H, Song J, He Y, et al. CRISPR/Cas9 screens reveal that Hexokinase 2 enhances cancer stemness and tumorigenicity by activating the ACSL4-fatty acid -oxidation pathway. Adv Sci Weinh 2022; 9: e2105126.
51.
Bartrons R, Simon-Molas H, Rodríguez-García A, et al. Fructose 2,6-bisphosphate in cancer cell metabolism. Front Oncol 2018; 8: 331.
52.
Simula L, Alifano M, Icard P. How phosphofructokinase-1 promotes PI3K and YAP/TAZ in cancer: therapeutic perspectives. Cancers (Basel) 2022; 14: 2478.
53.
Jin L, Chun J, Pan C, et al. Phosphorylation-mediated activation of LDHA promotes cancer cell invasion and tumour metastasis. Oncogene 2017; 36: 3797-806.
54.
Pu Z, Duda DG, Zhu Y, et al. VCP interaction with HMGB1 promotes hepatocellular carcinoma progression by activating the PI3K/AKT/mTOR pathway. J Transl Med 2022; 20: 212.
55.
Hwang SJ, Cho SH, Bang HJ, Hong JH, Kim KH, Lee HJ. 1,8-Dihydroxy-3-methoxy-anthraquinone inhibits tumor angiogenesis through HIF-1alpha downregulation. Biochem Pharmacol 2024; 220: 115972.
56.
Glaviano A, Foo ASC, Lam HY, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer 2023; 22: 138.
57.
Yu L, Wei J, Liu P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin Cancer Biol 2022; 85: 69-94.
58.
Sun EJ, Wankell M, Palamuthusingam P, McFarlane C, Hebbard L. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomedicines 2021; 9: 1639.
59.
Bamodu OA, Chang HL, Ong JR, Lee WH, Yeh CT, Tsai JT. Elevated PDK1 expression drives PI3K/AKT/MTOR signaling promotes radiation-resistant and dedifferentiated phenotype of hepatocellular carcinoma. Cells 2020; 9: 746.
60.
Tang Z, Zhao P, Zhang W, Zhang Q, Zhao M, Tan H. SALL4 activates PI3K/AKT signaling pathway through targeting PTEN, thus facilitating migration, invasion and proliferation of hepatocellular carcinoma cells. Aging (Albany NY) 2022; 14: 10081-92.
61.
Hong T, Dong D, Li J, Wang L. PARP9 knockdown confers protection against chemoresistance and immune escape of breast cancer cells by blocking the PI3K/AKT pathway. Arch Med Sci 2023; 20: 1228-48.
62.
Zong Z, Xie F, Wang S, et al. Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell 2024; 187: 2375-92.
63.
Song H, Chen L, Pan X, et al. Targeting tumor monocyte-intrinsic PD-L1 by rewiring STING signaling and enhancing STING agonist therapy. Cancer Cell 2025; 43: 503-18.e10.
64.
Zhang J, Ouyang F, Gao A, et al. ESM1 enhances fatty acid synthesis and vascular mimicry in ovarian cancer by utilizing the PKM2-dependent warburg effect within the hypoxic tumor microenvironment. Mol Cancer 2024; 23: 94.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.