Urinary incontinence (UI) is defined as “the complaint of any involuntary leakage of urine”, according to the International Continence Society (ICS). UI has profound effects on the social and physiological well-being of incontinent patients and caregivers [1], being associated with depression [2] skin damage [3], isolation, embarrassment, falls and fractures [4, 5]. UI increases with age due to the gradually failing ligamentous and connective tissue supporting the pelvic organs and decreasing volume and tone of pelvic muscles [6]. Overweight and obesity [7], impaired mobility and diabetes mellitus [8] are important risk factors for UI. Conservative management and lifestyle interventions are primary treatment options. Containment care using incontinence products, such as absorbing incontinence products (AIPs), is part of the standard of care [9]. To ensure the comfort of the incontinent individual, the caregiver often checks the saturation level of the AIP by touching, looking and/or asking.
A wetness indicator in the form of a strip that shows the absorption level has been on the market for some time. However, it still needs to be manually observed by the caregiver and requires undressing. Multiple electronic systems to detect urine saturation have been proposed [10] and there are also other devices [11–13] under development. Although the use and benefits are unclear, some improvement in the quality of continence care has been reported [14]. Furthermore, for UI due to neurogenic bladder, devices such as artificial urinary sphincters and neurostimulation are available [15].
In this study we investigated the TENA SmartCare Change Indicator (Change Indicator). The Change Indicator is a commercially available medical device manufactured by Essity Hygiene and Health AB (Gothenburg, Sweden). The device attaches to the outside of the AIP and consists of a reusable electronic sensor, transmitter and an application installed on one or more smart phones. The device estimates the wetness saturation of the AIP and communicates this to the caregiver via an app interface. The primary objective was to demonstrate that the device can reduce the number of manual AIP checks in a home care setting.
Methods
Study design and study population
The POWER trial (NCT04846270) was conducted in the Warsaw region, Poland, between April and December 2021. The trial included 35 elderly subjects with UI, cared for in a home environment. All subjects had used TENA incontinence products before as well as during the study.
The trial was performed over a period of three weeks. During the first (baseline) week, the device was not used. The device was introduced during the second (learning) week and the third (investigational) week. During the first and third week the number of manual checks was noted using a diary. Subjects attended five visits during the study.
Outcome measurements
The primary outcome was the number of daily manual checks during the investigational week compared to the baseline week. The secondary outcomes were the leakage on garments, skin redness/irritation, fecal incidences, usability, and safety. The source data were collected by caregivers using eDiaries (and/or paper version) and eQuestionnaires.
The variable change in skin redness/irritation was evaluated using the GLOBIAD [16] tool. At the end of the learning week and investigational week, caregivers were asked to evaluate the written instructions, the usability of the device and the application, performance of the investigated device, facilitation of decisions, well-being and comfort of subjects, overall usefulness, and continuous usage intention.
Statistical analysis
The investigational and baseline data were compared to discern any significant differences. The level of significance was set to 0.05 (p < 0.05) for all variables. Student’s paired t-test was used; if the variables were not normally distributed, the non-parametric Wilcoxon signed-rank test was used instead.
Results
Subjects of study
A total of 35 subjects completed the trial. There were no dropouts or withdrawals from the study. The mean age of the subjects was 74 years (SD, standard deviation, 23). 80% of subjects were female. Up to 85% of the study population experienced mild to severe cognitive impairment.
Primary and secondary outcomes
The mean number of daily checks decreased from 4.3 ±2.0 during the baseline week to 3.5 ±1.8 during the investigational week (Figure 1). The mean difference of 0.8 ±1.7 (or 15.9% ±26.0%) in number of checks per day was statistically significant (p = 0.0006 and p = 0.0010 respectively).
Figure 1
Comparison of number of checks, changes, leakages and fecal incidents between baseline and investigational weeks
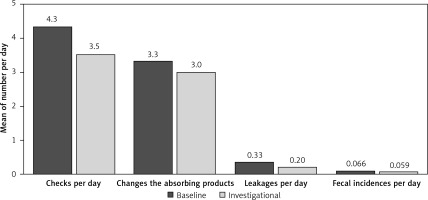
Daily leakages decreased from 0.33 ±0.44 during the baseline week to 0.20 ±0.37 during the investigation week (Figure 1). This was a statistically significant reduction of 0.13 ±0.29 (p = 0.0051) or 40%. Slight non-significant decreases in AIP changes and fecal incidents were also observed (Figure 1).
At the beginning of the baseline week, 5 out of 35 subjects (14%) showed skin redness/irritation. All were defined as “persistent redness without clinical signs of infection” and classified as the least severe grade (i.e., 1A) according to the GLOBIAD score. Two of the five subjects above were assessed with skin redness/irritation, scoring 1A, by the end of the baseline week. The other 33 subjects showed no skin redness/irritation. At the completion of the trial none of the 35 subjects showed signs of skin redness/irritation.
Safety endpoints
Five AEs were reported in four subjects. All AEs were considered unrelated to the medical device or the investigational procedures and none had the potential to become serious.
Thirty-nine device deficiencies (DDs) were reported in 27 subjects. These DDs mostly concerned problems with the sensor strip (29 cases). The source of these DDs was determined to be in a faulty batch of sensor strips that had a weakness in the cover material at the transmitter connection area. The DD did not affect the safety or performance of the sensor strip but affected its appearance. The remaining DDs concerned connectivity issues, meaning some disruptions in communication between the transmitter and the system.
Usability
74% of caregivers rated the written instructions, the use of the device (83%) and the app (91%) as ‘very easy’ (highest score) at the completion of the study. However, 29% of caregivers experienced obstacles with the device, and 14% faced obstacles with the app during the study.
Facilitation and well-being
At the end of the study 60% of caregivers reported an improvement in the subjects’ well-being and 71% of caregivers reported a reduction of worry about the comfort of the subject. Furthermore, 86% of caregivers answered that the system had facilitated the decision to change AIPs. 89% of the caregivers assessed the overall usefulness as ‘good’ or ‘very good’. 54% of the caregivers would continue to use the system if available on the market, and 89% would recommend the system.
Discussion
The use of the device appears to improve the physiological well-being of incontinent subjects and their caregivers. The reduction in manual checks was about 16%; we considered a 20% reduction in daily checks clinically significant, and the current data cannot rule out that this is the actual reduction. There was a reduction in the number of manual checks and leakages on garments and a slight decrease in the number of changes, fecal incidents, and skin redness/irritation. These findings imply that using the device can reduce the need for privacy invasion of incontinent subjects due to manual checks. Furthermore, the device appears to ensure that the aip will be changed at the right time, leading to subjects not needing to wear a wet aip for an extended period. That enhances the comfort and prevent subjects’ skin from prolonged exposure to urine and/or stool. Consequently, skin irritation/redness and/or incontinence-associated dermatitis such as fungal infections would be less likely to occur [3]. Although the device itself does not remedy the incontinence condition, its use could potentially increase the quality of life for subjects and caregivers. There are other commercial systems similar to the change indicator on the market, but to our knowledge there are limited clinical data available on their effectiveness. Experimental systems shows some promise to improve care in nursing home residents [17] but other data suggest there are still technical difficulties in achieving clinical benefit from these devices [18, 19]. The change indicator offers some advantages over current non-digital wetness indicators placed on the aips. The ability to get notified without physically having to check the product is beneficial. This allows the product to be changed at the discretion of the caregiver for instance if there is a concern about skin problems or distress of the user and not only because the product is full. Also, the caregiver and subject may have more undisturbed sleep, and during daytime caregivers have more freedom to perform other activities.
The system appeared to be safe and well tolerated. None of the five observed AEs were defined as severe and/or related to the medical device or the investigational procedures. Ultimately, the evaluation done by caregivers indicates that the investigated system is very easy to use. They are willing to use the system if available on the market and highly recommend others to use it.
Several limitations of the study should be addressed. Firstly, the study was open and single arm with a limited number of subjects. The observation period was short, even though one learning week appeared sufficient to establish a new baseline before the investigational week. Furthermore, data in the study were reported by the caregiver and could be subject to bias and differences in reporting.
In conclusion, the TENA SmartCare Change Indicator appears to be safe and well tolerated in its intended setting and in a home environment. It helps caregivers decide when to change the AIP, as demonstrated by the observed reductions in the number of manual checks and leakages. Overall usability aspects of device handling were reported to be satisfactory, and caregivers believe the system is easy to use and gives benefits to them and the comfort of the subjects.