Introduction
Antiplatelet treatment has proved to be effective in preventing bypass occlusion after coronary artery bypass grafting (CABG). Despite therapy, patients operated using a cardiopulmonary bypass showed increased platelet aggregation that rebounded to above preoperative levels [1, 2]. Considering the mechanism of early graft failure, it seems logical that potent platelet inhibition may improve graft patency [3–5]. By contrast, it is known that an impaired response to antiplatelet therapy underlies the increased risk of post-operative occlusions and can even result in death after CABG surgery [6]. Several potential explanations have been proposed for the nature of high on-treatment platelet reactivity, including modifiable factors: too low doses of anti-platelet drugs, high platelet turnover, oxidative stress, dyslipidaemia and inflammation [7–12]. Coronary artery bypass grafting surgery itself is associated with excessive activation of platelets, which results in stimulation of the production and release of young platelets [2, 13, 14].
It is noteworthy that platelet reactivity is strongly dependent on modifiable factors, especially on the inflammatory state [12, 15, 16]. The inflammation process, which is associated with CABG as a result of cardiopulmonary bypass, is affected by many factors, including surgical trauma, blood contact with the cardiopulmonary bypass (CPB) device’s artificial surfaces, as well as ischaemia and reperfusion injury. The pro-inflammatory cytokines interleukin (IL)-6 and IL-8, tumour necrosis factor-α (TNF-α), anti-inflammatory IL-10, and C-reactive protein (CRP) were found at increased levels following CABG [17].
Coronary artery bypass grafting surgery is associated with a systemic inflammatory response, endothelial damage and platelet activation, regardless of whether a CPB has been performed [18–20]. A number of publications indicate that this augmentation of platelet reactivity largely depends on a set of environmental factors, including the inflammatory state [15, 21]. The association between preoperative inflammatory parameters and early graft occlusion, as well as cardiovascular events following coronary artery bypass grafting, has not, however, been fully elucidated [22]. C-reactive protein is a powerful independent predictor of cardiovascular events in patients with coronary artery disease. Recent clinical trials targeting CRP have shown a reduction in major adverse cardiovascular events (MACE) after an acute coronary syndrome (ACS). Inflammation could be linked to high platelet reactivity (HPR), which is an independent predictor of MACE in patients with ACS [15]. A relation has been observed between CRP concentration and in-hospital outcome, following coronary artery bypass grafting [23]. It seems that a rise in the CRP level, as an inflammation marker, may be associated with transient platelet hyperactivity after CABG.
Therefore, the aim of this study was to evaluate the effect of an inflammatory state (measured by CRP, IL-6 and TNF-α levels) on platelet reactivity in a short postoperative period (6th day) using two different platelet assays and to analyse the results comprehensively.
Material and methods
Patients
In total, 140 patients with NSTE-ACS, who were not eligible for PCI and required urgent revascularization, were enrolled in the study (source population). All CABG procedures were performed at the First Department of Cardiac Surgery, Medical University of Silesia in Katowice (Poland). Patients meeting inclusion criteria were enrolled consecutively. Patients presenting at least two of the following criteria were included in the study: 1) symptoms of chest pain lasting for at least 20 min within a period of 24 h preceding the coronary angiography; 2) elevated troponin levels; 3) ST-segment depression of at least 0.1 mV or a negative T wave of at least 0.2 mV in two or more contiguous leads. All the patients enrolled in the study presented with complex coronary lesions including left main disease, multiple-vessel disease or a coronary anatomy not amenable to PCI, which was reflected by a SYNTAX score > 22 points (intermediate and high risk).
The predefined exclusion criteria were: chest pain longer than 48 h, ST segment elevation, oral pre-treatment with clopidogrel, age over 80 years, percutaneous revascularization procedure within the previous 30 days, CABG within the previous 6 months, NYHA class IV heart failure preceding hospitalization, gastrointestinal or genitourinary bleeding within the previous 30 days, history of intracranial disorders (including stroke, primary and metastatic brain tumours and history of head trauma); major surgery or high level trauma within the previous 6 weeks; history of bleeding diathesis, thrombocytopenia defined as less than 100,000 PLT/mm3, anticoagulation with INR ≥ 1.8, impairment of liver function, renal impairment with creatinine level > 1.5 mg%, uncontrolled blood pressure exceeding 200/110 mm Hg, and hypersensitivity to any drug.
The characteristics of the overall (source) population of the study (n = 140) were: female sex: 40 (28.6%) patients; age: 63.0 ±9.4 years; body mass index (BMI) 28.2 ±3.9 kg/m2; hypertension: 109 (77.9%); dyslipidaemia: 132 (94.3%); peripheral artery disease: 9 (6.4%); diabetes mellitus: 35 (25.0%); EuroSCORE: 5.6 ±2.0 points. All the patients were on aspirin until the day of the operation. A complete set of all clinical and laboratory data was gathered for the target group (n = 103). The characteristics of this group are presented in detail in Table I.
Table I
Characteristics of total group of coronary artery bypass grafting subjects and patients allocated to subgroups with high and low platelet reactivity (estimated using the platelet reactivity score) 6 days after the operation
| Variable | Whole group n = 103 | High platelet reactivity n = 43 | Low platelet reactivity n = 60 | P-value* |
|---|---|---|---|---|
| Female sex (%) | 35 (34.0) | 15 (34.5%) | 20 (33.3%) | ns |
| Age [years] | 62.7 ±9.9 | 61.0 ±10.9 | 63.1 ±9.4 | ns |
| BMI [kg/m2] | 27.7 ±3.3 | 28.7 ±3.5 | 27.6 ±3.2 | ns |
| Hypertension (%) | 85 (82.5) | 37 (86.0%) | 48 (80.0%) | ns |
| Dyslipidaemia (%) | 98 (95.1) | 41 (95.3%) | 57 (95.0%) | ns |
| Peripheral artery disease (%) | 8 (7.6) | 2 (4.7%) | 6 (10.0%) | ns |
| Diabetes mellitus (%) | 23 (22.3) | 12 (27.9%) | 11 (18.3%) | ns |
| EuroSCORE [points] | 5.8 ±2.0 | 6.0 ±2.2 | 5.7 ±1.9 | ns |
| Duration of CPB [min] | 87.4 ±21.6 | 85.4 ±26.2 | 88.3 ±23.3 | ns |
| Aorta clamping time [min] | 48.2 ±19.0 | 47.3 ±19.0 | 48.7 ±13.9 | ns |
| Postoperative blood drainage [ml] | 826 ±448 | 814 ±491 | 848 ±491 | ns |
| Need for PRBCs transfusion | 1.61 ±1.39 | 1.60 ±1.29 | 1.62 ±1.47 | ns |
| Need for FFP transfusion | 2.28 ±0.74 | 2.28 ±0.70 | 2.28 ±0.76 | ns |
| Need for PRP transfusion | 0.90 ±0.58 | 1.16 ±0.69 | 0.57 ±0.69 | ns |
| CK-MB [UI] | 24.00 (17.50–42.49) | 24.00 (16.50–52.50) | 24.00 (18.00–32.25) | ns |
| TnI [ng/ml] | 0.92 (0.38–2.01) | 0.99 (0.43–2.45) | 0.86 (0.37–1.95) | ns |
| Perioperative MI | 9 (8.7%) | 7 (16.3%) | 2 (3.3%) | < 0.04 |
| Postoperative rhythm disturbances | 13 (12.6%) | 9 (20.9%) | 4 (6.7%) | < 0.04 |
| Hb [g/dl] | 11.78 ±1.19 | 11.82 ±1.20 | 11.68 ±1.18 | ns |
| Leukocyte count [× 109/l] | 9.75 ±1.37 | 9.66 ±1.42 | 9.87 ±1.26 | ns |
| Platelet count [× 109/l] | 227.8 ±80.9 | 217.8 ±75.9 | 236.7 ±84.7 | ns |
| Mean platelet volume [fl] | 10.38 ±1.49 | 10.39 ±1.57 | 10.35 ±1.47 | ns |
| IL-6 [pg/l] | 22.8 (14.4–31.4) | 24.4 (14.8–37.5) | 21.4 (13.2–30.0) | ns |
| CRP [mg/l] | 54.1 (36.7–82.0) | 81.5 (44.7–104.8) | 44.6 (36.5–62.7) | < 0.02 |
| TNF-α [pg/l] | 2.59 (2.16–4.15) | 3.51 (2.38–5.25) | 2.37 (1.77–3.10) | < 0.02 |
Operative (surgery) techniques
Anaesthesia was induced and maintained using standard procedures. A median sternotomy was performed and patients were placed on CPB, under mild hypothermia at 34°C and cold cardioplegia at a 4 : 1 ratio to protect against myocardial ischaemia. Unfractionated heparin was delivered intravenously at a dose of 300 U/kg and then again every 60 min of CPB to maintain an activated clotting time of at least 480 s. Immediately after CPB was ceased, heparin was reversed with protamine sulphate at a dose of 3 mg/kg. Acetylsalicylic acid (aspirin) at a daily dose of 300 mg was reinitiated after the procedure, subsequently reduced to a daily dose of 150 mg on day 6 and maintained for the duration of the study. Clopidogrel at a daily dose of 75 mg was administered to each patient 6 days after the procedure.
Blood collection
Blood was collected by peripheral venipuncture into two plastic tubes (S-Monovette; Sarstedt, Nümbrecht, Germany). The first tube contained buffered 3.2% sodium citrate (Becton Dickinson, Plymouth, United Kingdom) with a final citrate to blood ratio of 1 : 9 vol/vol, while the second contained 250 μg/ml hirudin (Refludan, Schering AG, Germany), yielding a final concentration of 25 μg/ml in the blood sample. Whole blood was kept at room temperature. Blood samples for complete blood cell counts were collected in K2EDTA anticoagulated sample tubes. The samples were processed within 4 h.
Platelet reactivity
Determination of PFA-100 closure time
The closure time (CT expressed in seconds, maximal recorded value of 300 s) was measured using a PFA-100 flow analyser (Siemens Healthcare Diagnostics, Inc, Norwood, MA, USA). All blood samples were tested for closure times with collagen/epinephrine (CTCEPI) and collagen/ADP (CTCADP) cartridges in accordance with the manufacturer’s instructions, at least 30 min but not more than 1 h after the blood was withdrawn. PFA-100 measurements were performed in duplicate, and mean CT values were calculated.
Multiple electrode aggregometry
Whole blood aggregation was measured using a 5-channel, semi-automatic, dual measurement aggregometer (Multiplate Analyzer; Roche Diagnostics GmbH, Mannheim, Germany). The ADP Test (ADP; final concentration, 6.4 μmol) or Aspi-Test (arachidonic acid, final concentration, 0.5 mmol/l) was added as a platelet agonist, and an increase in electrical impedance caused by the growing platelet attachment to the electrodes was recorded continuously for 10 min. Platelet reactivity was expressed as the maximal aggregation (arbitrary units – AU).
Platelet reactivity score
In this paper, we suggest introducing a novel comprehensive score for assessing platelet reactivity: the platelet reactivity score (PRS). The rule for calculating PRS is shown below (medians are calculated on the basis of all the results of the given parameter (CTCEPI, CTCADP, ASPItest, ADPtest) in the studied group.
CTCEPI < median: 1; CTCEPI ≥ median: 0
CTCADP < median: 1; CTCADP ≥ median: 0
ASPItest > median: 1; ASPItest ≤ median: 0
ADPtest > median: 1; ADPtest ≤ median: 0
The PRS score range is from 0 to 4, with a cut-off platelet reactivity score of > 2. Patients were allocated to the high platelet reactivity (HPR) group or the low platelet reactivity (LPR) group (PRS ≤ 2).
Platelet activation
Immunoassays for soluble P-selectin (soluble CD62) and soluble CD40L
To obtain plasma for immunoassays, EDTA blood was prepared following standard procedures and the samples were stored at –50°C until analysis. Levels of sP-selectin and sCD40L in patients’ plasma were measured using commercially available ELISA tests (respectively, Human P-Selectin/CD62P DuoSet ELISA Quantikine and Human CD40 Ligand/TNFSF5 Quantikine ELISA Kit; R&D Systems, Minneapolis, Minnesota, USA). The intra-assay variability for the lower assay range was less than 10%. The measurement ranges were 0–50 ng/ml for sCD62 and 62–4000 pg/ml for sCD40L.
Inflammatory markers and other laboratory parameters
CK-MB, high-sensitive troponin I (hTnI) and high-sensitivity C-reactive protein (hs-CRP) serum levels were measured using an ELISA test. To assess qualitative and quantitative changes in platelets the following parameters were analysed: mean platelet count (MPC), mean platelet volume (MPV) and large platelet concentration ratio (L-PCR). Cell blood counts were carried out using the Sysmex XE-2100 (Sysmex Corporation, Kobe, Kansai, Japan).
Immunoassays for tumour necrosis factor-α and interleukin-6
To obtain serum for immunoassays, non-anticoagulated blood was prepared following standard procedures and samples were stored at –50°C until analysis. Levels of TNF-α and IL-6 in patients’ serum were measured using commercially available ELISA tests (respectively, Human TNF-alpha Quantikine ELISA Kit and Human IL-6 Quantikine ELISA Kit, R&D Systems, Minneapolis, Minnesota, USA). The intra-assay variability for the lower assay range was less than 10%. The measurement ranges for TNF-α were 0.5–32 pg/ml and 3.1–300 pg/ml for IL-6.
Measurement points
Two time points were used to test all parameters: (T1) before CABG, (T2) 6 days after the surgery procedure.
Statistical analysis
Mean ± SD are given for normally distributed variables. Medians and interquartile ranges (Me, IQ) are given for other parameters showing departures from normality (according to the Shapiro-Wilk W test). Simple paired comparisons were performed using unpaired Student t test, while non-normally distributed variables were analysed using the Mann-Whitney U test. Pearson’s χ2 test with Yates correction (if necessary) was used for categorical data. Cohen’s k coefficient was calculated as a measure of agreement. A k statistic value of < 0.4 represents poor-to-fair agreement, a value of 0.41–0.60 reflects moderate agreement, a value of 0.61–0.80 is considered good agreement, and a k value of 0.81–1.0 is considered very good agreement. The odds ratio (OR) and 95% confidence interval (CI) were assessed to establish the correlation between PRS and inflammatory markers using logistic regression.
The statistical analysis was performed using Statistica 13.1 (StatSoft Inc., Tulsa, Oklahoma).
Results
Patients and procedural characteristics
Thirty-seven patients were excluded from the study due to incomplete laboratory parameters. In total, 103 NSTE-ACS patients, requiring urgent coronary artery bypass graft surgery, were included in the study. The characteristics of selected parameters in CABG patients before and 6 days after the operation are presented in Table II.
Table II
Characteristics of selected parameters of coronary artery bypass grafting patients (n = 103) before and 6 days after operation
[i] Continuous data are presented as mean ±SD or as median (interquartile range: LQ–UQ). Categorical data are presented as absolute numbers (n) and %. Significance of differences was analyzed using Mann-Whitney U test (continuous data) or Fisher’s exact test (categorical data). Hb – hemoglobin, Tnl – troponin I, CRP – C-reactive protein, TNF-α – tumour necrosis factor-α, IL-6 – interleukin-6.
Platelet reactivity and activation on the 6th day after the operation
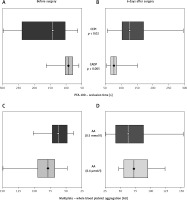
Platelet reactivity significantly increased in the postoperative period, as reflected by CTCEPI and CTCADP. In the case of arachidonic acid (ASPItest) and ADP-induced aggregation (ADPtest), there were no differences between the pre-operative period and on the 6th day after CABG (Figure 1).
Figure 1
Platelet reactivity monitored in coronary artery bypass grafting (CABG) patients using PFA-100 and Multiplate (MEA – multiple electrode aggregometry) on the day before surgery and 6th day after surgery. Data shown as median (interquartile range, minimum and maximum values). Significance of differences was analysed using the Mann-Whitney U test
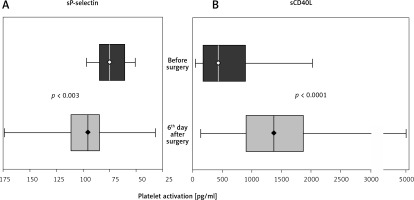
Platelet activation significantly increased in the post-operative period, as reflected by the elevated levels of soluble P-selectin and soluble CD40L (Figure 2).
Figure 2
Platelet activation monitored in coronary artery bypass grafting (CABG) patients using immunoassays for soluble P-selectin and soluble CD40L on the day before surgery and 6th day after surgery. Data shown as median (interquartile range, minimum and maximum values). Significance of differences was analysed using the Mann-Whitney U test
High platelet reactivity and low platelet reactivity groups
There was no association between individual parameters (analysed alone) of platelet reactivity dependent on aspirin (CTCEPI, ASPItest) and a very low association between parameters of platelet reactivity dependent on P2Y12 inhibitors (CTCADP, ADPtest) and inflammatory markers. However, in the next approach, we used a complex, comprehensive score (the novel PRS) and divided the whole group into high platelet reactivity (PRS > 2; HPR; n = 43) and low platelet reactivity (PRS ≤ 2; LPR; n = 60) subgroups. Using this approach, the elevation of two parameters, CRP and TNF-α, was found in the HPR subgroup in comparison with the LPR group of CABG patients (Table I).
Perioperative outcomes
Perioperative myocardial infarction and rhythm disturbances occurred more frequently in the high platelet reactivity group (differences close to statistical significance). Post-operative chest bleeding, prolonged ventilator use, renal failure and stroke were similar in both groups. Levels of cardiac markers, EuroSCORE and other clinical parameters were comparable in both tested groups (Table I).
Agreement and regression analysis of selected inflammatory markers with platelet reactivity or activation on the 6th day after CABG
A significant association was found between CRP, TNF-α, IL-6 and platelet reactivity (platelet reactivity score) but not with platelet activation (sCD40L, sP-selectin). The agreement between the results was assessed using Cohen’s k test and is presented in Table III. Concordance between platelet reactivity and inflammatory markers was in the poor to fair range but reached a level of statistical significance.
Table III
Agreement of selected inflammatory markers with platelet reactivity on 6th day after coronary artery bypass grafting
| Markers | Observed agreement [%] | Cohen’s k | Confidence interval (95% CI) | P-value |
|---|---|---|---|---|
| CRP | 74.5 | 0.49 | 0.246–0.738 | < 0.0001 |
| TNF-α | 68.3 | 0.37 | 0.019–0.612 | < 0.002 |
| IL-6 | 61.9 | 0.24 | –0.011–0.432 | < 0.03 |
A logistic regression analysis showed that CRP and TNF-α were associated with platelet reactivity (Table IV).
Discussion
The inflammatory milieu that occurs after CABG as a result of cardiopulmonary bypass has been extensively studied and is the consequence of multiple factors, including surgical trauma, and blood contact with the foreign surfaces of the CPB [5, 17]. On the other hand, the relationship between inflammatory state, CABG and high platelet reactivity has rarely been discussed [20, 24]. Therefore, the aim of this study was to analyse the association between platelet reactivity or platelet activation after CABG with selected markers of the inflammatory state. In our work, the simultaneous use of Multiplate and PFA-100 analysers and the PRS proved to be useful tools for detecting a connection between platelet reactivity and an inflammatory state.
Literature reports that high platelet reactivity after CABG has an incidence range of 10–90% [21, 25]. This is a transient phenomenon, more frequently observed during the first month after surgery, and some authors suggest that it may explain the elevated risk of graft thrombosis and death after CABG [2, 21]. The response of platelets to CPB is complex, with a reduction in platelet count from a baseline during the first days, and then increasing to a higher count than before surgery [1, 13, 26, 27]. Cardiopulmonary bypass also causes a systemic inflammatory response and platelet activation [26]. Plasma levels of IL-6 significantly correlate with a systemic inflammatory response and reflect the severity of acute inflammation [24].
In this study, we found that the increased platelet reactivity was significantly associated with high levels of CRP, TNF and IL-6, but only when platelet reactivity was expressed as a complex PRS. We observed a significant increase in CRP level during the first week after CABG, peaking at the same time as the lowest anti-platelet therapy effectiveness was recorded. This phenomenon might account for the increased risk of occlusion of bypass grafts at this moment during the post-operative period [28, 29]. Measurements of residual platelet reactivity by PFA-100 [28] and whole blood platelet aggregometry [30] are considered adequate for predicting risks of cardiovascular events.
We found that perioperative myocardial infarction and rhythm disturbances occurred more frequently in the high platelet reactivity group. By contrast, basal platelet activation was not associated with inflammatory markers in this study. In the literature, perioperative myocardial infarction and rhythm disturbances were observed but they have rarely been correlated with the inflammatory state and platelet reactivity [31–33]. Interestingly, Plicner et al. showed that clinical outcomes in the early postoperative period are associated with inflammation and platelet activation markers [20].
In our study, all patients displayed a rise in platelet reactivity, despite continuing the same antiplatelet treatment after surgery. The reduced antiplatelet effect of aspirin is associated with low-grade inflammation in patients with coronary artery disease [34]. In our study, we observed a similar tendency in CABG patients. Recent literature has confirmed that CRP promotes platelet activation [35]. Additionally, CRP was found in platelet aggregates and stimulated further platelet deposition at the arterial wall [36]. Moreover, an association was also observed between increased IL6 levels and increased platelet count.
In this study, we demonstrated the platelet hyperreactivity as measured by PFA-100 CTCADP (cartridges with ADP and collagen) and by aggregometry (multiplate with ADP as agonist). Interestingly, Gluckman et al. suggested platelet hyperreactivity measured by PFA-100 CTCADP to be a novel independent risk factor for early thrombosis after CABG surgery [28].
In our opinion, application of the PRS with a cut-off at PRS > 2 indicating platelet hyperactivity allows one to investigate complex dependencies between platelet reactivity and inflammatory state after CABG despite a small number of patients. We believe that the association between inflammatory state and platelet hyperreactivity as well as perioperative outcomes, demonstrated in this study, suggests a need of a combined analysis of both platelet reactivity and inflammatory markers (in particular CRP) in order to assess perioperative outcomes.
In conclusion, our study demonstrated that increased platelet reactivity, expressed as a novel PRS after CABG, is associated with a higher concentration of inflammation markers. Furthermore, following a cardiac operation with extra-corporeal circulation, platelet hyperreactivity in the early post-operative period, combined with a systemic inflammatory state, correlates with a higher risk of post-operative rhythm disturbances.