Introduction
Acute variceal hemorrhage is a major complication of liver cirrhosis and is responsible for one third of cirrhosis deaths [1, 2]. The Child-Turcotte-Pugh score and the Model for End Stage Liver Disease (MELD), proven to have a prognostic value in liver cirrhosis [3, 4], have also been validated for the clinical course of variceal hemorrhage [5−7].
Although insufficiently validated, the presence of comorbidities cannot be ignored when discussing short-term mortality. Some research groups have already included in mixed scores the different variables that seem to influence the prognosis of liver cirrhosis patients (CirCom) [8].
On the other hand, the recent progress in the therapeutic means for variceal hemorrhage (band ligation, vasoactive medication, antibiotic treatment) [9−14] has led to better control of bleeding; as a result, the natural history and prognosis of these patients can be different from the data published previously. The studies report values of mortality between 16% and 24% 6 weeks after the bleeding episode; these values have improved in the past few years but are still high enough to be disturbing and to motivate the continuing research on the risk factors and treatment. The key moment to calculate the prognosis is 6 weeks after the variceal hemorrhage, since the risk of death of patients after this threshold becomes similar to that of patients who have never bled [2, 15, 16].
The aim of this prospective, observational study was:
Material and methods
Patient selection criteria
We included in the study all consecutive patients presenting to the Emergency Department of our tertiary medical centre (the Regional Institute of Gastroenterology and Hepatology “Prof. Dr. Octavian Fodor”, Cluj-Napoca, Romania) with upper gastrointestinal bleeding (UGIB) of variceal origin between November 2012 and July 2013. It is worth noting that our hospital is the reference centre for the endoscopic treatment of upper digestive bleeding in the entire region (including almost 7 million inhabitants). All patients were prospectively followed over a period of 6 weeks. The inclusion criteria were the following: liver cirrhosis diagnosis, hematemesis and/or melena, bleeding of variceal origin, age above 18 years and informed consent signed. Patients having different complications of cirrhosis, including hepatocarcinoma, were not excluded from the study.
Cirrhosis was diagnosed using unequivocal clinical (palmar erythema, spider nevi, gynecomasty, hepatomegaly with sharp anterior margin, splenomegaly, ascites, edema, encephalopathy), laboratory and imaging criteria (irregular liver contour, splenomegaly, ascites, perigastric and pericholecystic collateral circulation and in the spleen hilum, recanalization of the round ligament). Acute kidney injury (AKI) was defined as serum creatinine ≥ 1.5 mg/dl according to the existing guidelines at the moment of inclusion [17].
The study was performed in accordance with the Helsinki declaration and was approved by the Ethics Committee of the Hospital. All patients signed an informed consent form.
Patient management
Demographic and clinical data considered relevant for liver cirrhosis were recorded for each patient upon admission, as well as their comorbidities. Hemodynamic instability was defined as either arterial hypotension (blood pressure below 90 mm Hg) or tachycardia (heart rate above 100 beats/min).
Esophagogastroduodenoscopy was performed in each patient in the first 12 h with an Olympus Exera II CLE165 device. Because of the hemodynamic instability in 4 cases endoscopy was performed within 24 h (between 11 and 19 h). After confirming the variceal site of the bleeding, band ligation and/or sclerotherapy (for gastric varices) were performed. In case of failure to control bleeding (FTB) or rebleeding, the endoscopy was repeated, as well as the band ligation. The balloon tamponade (n = 13) was used in the case of massive bleeding where an effective endoscopic treatment could not be performed. Beside the endoscopic treatment, all patients received vasoactive medication (Sandostatin 50 μg bolus followed by continuous infusion of 50 μg/h or terlipressin 2 mg i.v. bolus followed 1 mg every 6 h, for up to 5 days) and all patients received antibiotic treatment (IV 3rd generation cephalosporins for at least 5 days). Secondary prophylaxis was started on the 6th day after controlling the bleeding episode and was performed using a non-selective β-blocker (propranolol with a starting dose of 40 mg/day and titrated up to 120 mg/day) treatment and endoscopic band ligation.
When assessing comorbidities, we used both the CirCom [8] score, which quantifies conditions with an impact on cirrhosis progression, and the Charlson comorbidity index [18], which quantifies conditions with an impact on any other disease. Considering that all patients had liver cirrhosis, in calculating the Charlson index all patients were attributed at least 3 points. For each of the 2 scores used, the patients were divided into 2 groups: without comorbidities and with at least one comorbidity.
Patient follow-up
All patients were followed for 6 weeks. According to the Baveno V recommendation, the time interval used to define the acute bleeding episode was 5 days and failure to control bleeding was defined as follows: death or need to change therapy defined by one of the following criteria:
Statistical analysis
The statistical analysis was performed using the SPSS software, version 20, Chicago, IL, USA. Nominal variables were characterized using frequencies. Quantitative variables were described by mean and standard deviation or by median and IQR, as appropriate. The level of statistical significance was set at p < 0.05. Differences of frequencies between nominal variables were assessed with the χ2 test or Fisher exact test. Continuous variables were compared using the Student t or Mann-Whitney test, as appropriate. Multivariate analysis was performed using logistic regression. We included in the univariate analysis the parameters reaching a significance level p < 0.05. The cutoff value was chosen for maximal sensitivity and specificity. We calculated the sensibility, specificity, positive predictive value and negative predictive values for the cutoff value of the score.
Results
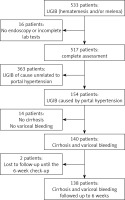
Between November 2012 and July 2013, 533 patients with upper gastrointestinal bleeding (hematemesis and/or melena) presented to the Emergency Department of the Regional Institute of Gastroenterology and Hepatology “Prof. Dr. Octavian Fodor”. Of these, 16 did not undergo upper digestive endoscopy and were excluded from the study. In 154 patients, the cause of the UGIB was related to the portal hypertension, but 14 did not have cirrhosis or the origin of the bleeding was other than esophageal varices. The remaining 140 patients had both liver cirrhosis and variceal bleeding. Two patients were lost from the study until the 6-week check-up. It follows that 138 patients were included in the final analysis (Figure 1). Among them, 49 (35.5%) had previous variceal bleeding and were under secondary prophylaxis with nonselective β-blocker (NSBB) and endoscopic band ligation.
Baseline characteristics of the patients included in the study are presented in Table I. The male gender was dominant, with a median age of 58 years; the ethanolic etiology was highly prevalent, 74 (53.6%) were at the first episode of variceal hemorrhage, 51 (37%) patients had hepatic encephalopathy, 74 (53.6%) had ascites, 13 (9.42%) had hepatocarcinoma. The anemia found in patients was on average of moderate severity; patients needed a total quantity of 34 units of blood, with a mean of 2.71 ±2.6. At the inclusion in the study, 16 (11.59%) patients had AKI (creatinine > 1.5 mg/dl). We found an equal distribution between Child B and C patients.
Table I
Baseline characteristics of study group
Failure to control bleeding
In our study group, 27 patients (19.5%) were considered to have failure of bleeding control. Sixteen (59.27%) patients of those with uncontrolled hemorrhage died, but all had been hemodynamically unstable at presentation. Eight of them had fresh hematemesis. A decrease of > 3 g in hemoglobin 48 h from admission was found in 3 (11.11%) patients. Blood transfusion had a significant association with the control of bleeding (2.40 ±2.1 vs. 4.21 ±2.85 in FTB group; p = 0.02). Of the comorbidities, diabetes mellitus was present in 28 patients in the whole study group, only 3 patients from FTB group having diabetes. The etiology of cirrhosis had no influence on variceal bleeding control (p = 0.11). The factors associated with failure to control bleeding are presented in Table II.
Table II
Univariate analysis associated with failure to control bleeding
| Variable | Failure to control bleeding | P-value | |
|---|---|---|---|
| Yes (n = 27) | No (n = 111) | ||
| First episode of bleeding, n (%) | 21 (77.77) | 68 (61.26) | 0.16* |
| Arterial hypotension, n (%) | 3 (11.11) | 5 (4.50) | 0.39* |
| Tachycardia, n (%) | 16 (59.25) | 42 (37.83) | 0.07* |
| Hemodynamic instability, n (%) | 17 (62.96) | 45 (40.54) | 0.05* |
| Active bleeding at endoscopy, n (%) | 4 (14.81) | 30 (27.02) | 0.18* |
| High risk of FTB§ | 0 (0) | 12 (10.81) | 0.15* |
| Creatinine [mg/dl] | 1.04 (0.73; 1.95) | 0.81 (0.60; 1.32) | 0.01** |
| Creatinine > 1.5, n (%) | 7 (25.92) | 9 (8.10) | 0.02* |
| Total bilirubin [mg/dl] | 4.10 (1.60; 10.00) | 2.70 (1.47; 6.10) | 0.15** |
| Albumin [g/dl] | 2.60 (2.32; 2.95) | 3.10 (2.60; 3.40) | 0.03** |
| INR | 2.13 (1.59; 3.04) | 1.83 (1.51; 2.22) | 0.10** |
| Prothrombin time (s) | 30.70 (22.15; 41.25) | 25.00 (22.00; 31.00) | 0.14** |
| Ascites, n (%) | 17 (62.96) | 54 (48.64) | 0.38* |
| Hepatic encephalopathy, n (%) | 18 (66.66) | 33 (29.72) | < 0.001* |
| Child class, n (%): | |||
| A | 2 (7.40) | 25 (22.52) | |
| B | 5 (18.51) | 50 (45.04) | < 0.001* |
| C | 20 (74.07) | 36 (32.43) | |
| MELD | 20.5 (16.00; 26.00) | 16 (13.00; 19.00) | 0.004** |
| Charlson | 3 (3; 7) | 3 (3; 6) | 0.10¶ |
| Charlson ≥ 4, n (%) | 1 (3.70) | 9 (8.10) | 0.70* |
| CirCom | 0 (0; 2) | 0 (0; 1) | 0.41¶ |
| CirCom ≥ 1, n (%) | 4 (14.81) | 24 (21.62) | 0.60* |
According to the clinical criteria of patients defined as high risk for failure to control bleeding (Child C or Child B plus active bleeding) [19], in our population, only Child C class of severity significantly predicted the FTB (20 (74.07%) vs. 36 (32.43%), p = 0.002). Child B patients bleeding actively during endoscopy did not have a higher rate of FTB (p = 0.15); nor did Child B plus C patients who presented active bleeding (16 (59.25%) vs. 44 (39.63%), p = 0.10).
Time from symptoms onset to endoscopy was significantly shorter in patients with FTB (9.24 (6.60–17.16)) vs. patients without FTB (14.70 (7.36–28.25)), p = 0.04, due to the more rapid transfer of severe patients to the endoscopy department. In multivariate analysis, only the Child class was significantly associated with failure to control bleeding, which is a marker of the real influence of the severity of the main disease. When the time from admission in the hospital to endoscopy was analyzed, we found no difference between patients with and without FTB (2.48 (1.30–4.62) vs. 1.72 (1.23–3.38)), p = 0.17. The difference is created by the interval between symptoms onset and admission in the hospital, which is significantly shorter in severe patients: 8.77 (4.63–24.00) vs. 13.22 (4.90–25.00), p = 0.09. Patients with a lower Charlson score had a higher rate of FTB without reaching statistical significance (1 patient from the group with failure vs. 9 patients from the group without failure, p = 0.47), suggesting that comorbidities are less important for bleeding control than the severity of the disease.
As mentioned before, in multivariate analysis, only the severity of the disease expressed through Child class was independently associated with failure to control bleeding (Table III). In order to avoid collinearity, we did not include encephalopathy, ascites or INR in multivariate analyses, since they are variables of the Child-Pugh score.
Forty-two day mortality
The next objective was to analyze the factors associated with 42-day mortality. Thirty (21.7%) patients died during the first 6 weeks: 19 (63.3%) from uncontrolled bleeding, 9 (30%) due to hepatic failure and 2 (6.6%) due to ventricular fibrillation. As mentioned, 16 (53.33%) patients died in the first 5 days.
The factors associated with 42-day mortality are presented in Table IV.
Table IV
Univariate analysis of factors associated with 42-day mortality
| Variable | 42-day check-up | P-value | |
|---|---|---|---|
| Dead (n = 30) | Survived (n = 108) | ||
| First episode of bleeding, n (%) | 23 (76.66) | 66 (61.11) | 0.17* |
| Arterial hypotension, n (%) | 3 (10) | 5 (4.6) | 0.50* |
| Tachycardia, n (%) | 19 (63.3) | 39 (36.1) | 0.01* |
| Active bleeding during endoscopy, n (%) | 10 (33.3) | 27 (25) | 0.49* |
| High risk of FTB§, n (%) | 1 (3.33) | 11 (10.18) | 0.41* |
| Creatinine [mg/dl] | 1 (0.7; 1.6) | 0.7 (0.6;1) | 0.02** |
| Creatinine > 1.5, n (%) | 8 (26.66) | 8 (7.40) | < 0.001* |
| Total bilirubin [mg/dl] | 3.9 (2.2; 6.2) | 2.0 (1.3; 4.0) | < 0.001** |
| Albumin [g/dl] | 2.5 ±0.5 | 3.00 ±0.7 | 0.001*** |
| INR | 2.14 (1.66; 2.66) | 1.60 (1.49; 1.89) | 0.001** |
| Prothrombin time [s] | 32.45 (26.9; 42.8) | 24.50 (21.8; 29.3) | 0.002** |
| Ascites, n (%) | 20 (66.7) | 79 (73.2) | 0.64* |
| Hepatic encephalopathy, n (%) | 21 (70) | 30 (27.8) | < 0.001* |
| Child class, n (%): | |||
| A | 1 (3.33) | 26 (24.07) | |
| B | 6 (20.00) | 49 (45.37) | < 0.001* |
| C | 23 (76.66) | 33 (30.55) | |
| MELD score | 21 (17.5; 27.5) | 16 (13; 19) | < 0.001** |
| Charlson index | 3 (3; 7) | 3 (3; 6) | 0.30¶ |
| Charlson > 4, n (%) | 11 (36.7) | 51 (47.2) | 0.41* |
| CirCom | 0 (0; 2) | 0 (0; 1) | 0.54¶ |
| CirCom > 1, n (%) | 5 (16.7) | 23 (21.3) | 0.76* |
| Failure to control bleeding, n (%) | 20 (66.66) | 5 (4.62) | < 0.001* |
Again, the most important factors associated with 42-day mortality are those related to the severity of the disease. The MELD score and the Child class were significantly associated in univariate analysis with death at 6 weeks after UGIB (p < 0.001 in both cases). We found the MELD > 18 cutoff to be predictive of death (sensitivity 69%, specificity 72.6%).
It is worth noting that AKI had a significant influence on 6-week survival (p = 0.009). In addition, AKI was significantly correlated with the presence of ascites (p = 0.01). Failure to control bleeding at 5 days was also significantly associated with 42-day mortality (p < 0.001). Of the patients defined as high risk for failure to control bleeding, only the Child C severity class had a significant influence on mortality prediction (p = 0.001). Patients from Child B class with active bleeding at endoscopy did not have a higher mortality risk in our study (p = 0.41), only when considering Child B plus C with active bleeding together (20 (66.66%) vs. 40 (37.08%), p = 0.007).
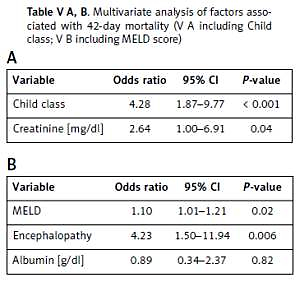
In order to avoid data collinearity, for multivariate analysis, we created two different models, one of them including the Child class while the other included the MELD score. In both models, we did not include the variables used for calculating Child and MELD scores, but only the score values, so as not to insert the variables twice in the analysis. In the first model, including the Child class, only the severity of the disease expressed by the Child score was independently associated with mortality at 6 weeks (p = 0.0005, OR = 4.28, 95% CI: 1.87–9.77), while in the second model, the MELD score (OR = 1.10, 95% CI: 1.01–1.21, p = 0.02) and encephalopathy (OR = 4.23, 95% CI: 1.50–11.94, p = 0.006) were independently associated with mortality (p = 0.02 and p = 0.006 respectively; Table V A, B).
Table V A, B
Multivariate analysis of factors associated with 42-day mortality (V A including Child class; V B including MELD score)
| A | |||
|---|---|---|---|
| Variable | Odds ratio | 95% CI | P-value |
| Child class | 4.28 | 1.87–9.77 | < 0.001 |
| Creatinine [mg/dl] | 2.64 | 1.00–6.91 | 0.04 |
| B | |||
|---|---|---|---|
| Variable | Odds ratio | 95% CI | P-value |
| MELD | 1.10 | 1.01–1.21 | 0.02 |
| Encephalopathy | 4.23 | 1.50–11.94 | 0.006 |
| Albumin [g/dl] | 0.89 | 0.34–2.37 | 0.82 |
Discussion
The present study proves that comorbidities (quantified using the Charlson and CirCom scores) do not influence the short-term prognosis of patients with variceal bleeding. The highest prognostic value is held by the liver function (Child and MELD scores) but the occurrence of AKI should definitely not be neglected. We find the validation of the prognostic factors for patients with variceal bleeding on an Eastern European population to be essential, when we consider the geographical, genetic and lifestyle differences, which greatly influence the etiology of liver cirrhosis and the pathology of variceal bleeding [20, 21].
When assessing the 6-week mortality, the impossibility to control bleeding in the first days after the bleeding episode is of the utmost importance. Several studies have analyzed the factors associated with 5-day rebleeding or continued bleeding; some of these include active bleeding at endoscopy [14, 22, 23], variceal size [24], Child-Pugh class [12, 14, 22, 24, 25], hematocrit level [14, 26], bacterial infections [22, 26], hepatic encephalopathy [27], portal vein thrombosis [14], the presence of hepatocellular carcinoma [26], and hypoalbuminemia [22]. Our study confirms these data (Table II). Lately, there is a debate regarding the most important prognostic factors that would indicate the need for further interventions, such as transjugular intrahepatic portosystemic shunt, and according to the latest evidence, Child-Pugh C has better prognostic relevance [28, 29] than Child-Pugh B and active bleeding at endoscopy. Our real life cohort confirmed these findings although the patients were not specifically evaluated for early TIPS insertion. Active bleeding was not associated with either FTB or 42-day survival, unlike Child-Pugh score, which was independently associated with both end-points.
However, contrary to the previous reports, we did not find an influence of comorbidities (CirCom and Charlson indices) on the failure to control bleeding. Our results may be biased due to the small number of patients in the comorbidity group and to the low comorbidity scores. Nevertheless, it is important to mention that AKI has a significant influence on the failure to control bleeding. Both the creatinine level per se and the association of kidney failure (defined as a creatinine level above 1.5 mg/dl) were associated significantly with rebleeding at 5 days, possibly due to the severity of bleeding as well as that of the main condition. This particular fact has had little coverage in the literature so far. On the other hand, the correlation between kidney injury and ascites during liver cirrhosis is well known.
Among the factors associated with both end-points, we found that creatinine is strongly and independently associated with 42-day mortality in the model that did not include the MELD score. This finding is in concordance with other data, where renal failure is associated with a 7-fold increase in mortality, with half of patients dying in the first month [30]. After the recent publication of the new criteria of AKI [31], which discourages the use of the 1.5 mg/dl threshold for serum creatinine, the biggest challenge is to diagnose AKI in the absence of baseline serum creatinine. In our cohort, in the absence of baseline serum creatinine levels, we successfully identified patients with higher mortality using the 1.5 mg/dl threshold. One of the limitations of our study is that, in the absence of infectious symptoms, the included patients had no extensive infectious work-up at the inclusion, and thus we cannot analyze the relation between AKI and bacterial infections, both associated with bad prognosis in patients with variceal bleeding [32]. However, as the guidelines recommend [33] all patients received prophylactic antibiotherapy and none presented persistent renal failure at discharge. We previously demonstrated that presence of AKI and bacterial infections were independently associated with in-hospital mortality in patients with decompensated cirrhosis (mainly ascites) [34]. Although there is strong evidence that bacterial infections frequently determine AKI, we believe that we cannot speak only about a cause-effect relation between AKI, bacterial infections and survival.
The significant difference in the time interval between the apparent bleeding and the moment of the endoscopy deserves to be mentioned. From our knowledge there are limited data regarding the relation between time intervals (from symptoms onset to endoscopy and from admission to hospital to endoscopy) and the prognosis of the patients with variceal bleeding. Time from symptoms onset to endoscopy was significantly shorter in patients with FTB, due to the more rapid transfer of severe patients to the endoscopy department. When time from admission to endoscopy was analyzed we found no difference between patients with and without FTB, demonstrating that in a tertiary health care center, the recommendation of rapid endoscopy (in the first 12 h) is followed. The difference is created by the interval between symptoms onset and admission to the hospital, which is significantly shorter in severe patients. In multivariate analysis, only the Child class was significantly associated with failure to control bleeding, which is a marker of the real influence of main disease severity. Therefore, Child-Pugh class C may be used as an indicator for rapid transfer to a 24-hour endoscopy facility.
At the 6-week check-up, the mortality in our group was 21.47%. Our results are similar to those reported by d’Amico et al., but higher than the values of 14.5–18.4% reported in other studies [10–12, 35].
In concordance with the previous reports, in our study the factors associated with 42-day mortality were mainly those depending on the liver function, such as the Child-Pugh [12, 25, 36, 37] and MELD scores [38, 39], total bilirubin [40, 41], INR [40, 42], ascites [41], hepatic encephalopathy [25, 43] or albumin [44]. Regarding the endoscopic risk factors, we failed to demonstrate any association between active bleeding at endoscopy and FTB or 42-day death. However, as expected, the patients with FTB had higher 42-day mortality and the hemodynamic instability as a sign of severe bleeding was correlated with FTB, similar to previous reports [11, 12].
The factors most strongly associated with the end-points were the Child-Pugh and MELD score, and among the variables used in their calculation, the largest impact was exerted by creatinine for MELD and encephalopathy for the Child-Pugh score. Only encephalopathy among the Child-Pugh variables (OR = 3.84, 95% CI: 1.44–10.25, p = 0.007) and only creatinine among the MELD score variables (OR = 3.65, 95% CI: 1.40–9.51, p = 0.008) were independently associated with 42-day mortality. The MELD score was proven to have the highest discriminative value for 42-day mortality prediction, with the best cut-off of 19 [5], which is very similar to our cut-off value of 18. On the other hand, the Child score remains widely used in clinical practice and research, for being superior to other scores studied for the prediction of mortality 6 weeks after variceal bleeding [25, 45, 46]. It is worth noting that both the score per se as well as its variables were identified as predictive factors for bleeding [47]. The score is easy to use at the bedside, requiring just a simple mathematical calculation, but has some disadvantages related to the subjective assessment of encephalopathy and ascites severity. However, it is generally accepted that liver function is the most important factor associated with short-term prognosis after variceal bleeding.
In our population, we did not observe any association between comorbidities quantified by the Charlson and CirCom scores and the 42-day prognosis. However, these score were not created to predict short-term prognosis and, moreover, the Charlson score is not intended for patients with cirrhosis. Acute kidney injury already present at admission appears to be an important prognostic factor and the nutritional status seems to be also important [48], but more studies are needed to confirm these findings. In addition, new diagnostic criteria need further validation in the setting of variceal bleeding.
This study has certain strengths and limitations. It reports the experience of a single referral center, which may be considered a limitation, but our institute is the referral center for treating variceal bleeding in a large region. Furthermore, the data were included prospectively and the patients were carefully followed for at least 6 weeks and therefore we consider the data to be representative for the Eastern-European region. On the other hand, we are aware of the absence of minimally invasive therapy of urgent decrease of portal hypertension (early TIPS), and consequently, this study has analyzed only the factors associated with mortality and rebleeding in patients treated conservatively. In this context, the hepatic venous pressure gradient (HVPG) was not assessed in our patients, so as to evaluate its influence on the failure to control bleeding and on mortality. Another limitation of the study is the lack of baseline serum creatinine values (before the bleeding) in order to apply the new diagnostic criteria of renal dysfunction [31] and to better quantify the influence of renal dysfunction on prognosis.
In conclusion, the severity of cirrhosis is an important prognostic factor for FTB and 42-day mortality. Despite the substantial improvements made in the therapy of variceal bleeding, mortality remains high, especially in severe patients [49]. Identifying the factors associated with rebleeding and early mortality may help in selecting from the beginning the patients in need of more than just conventional therapy. New studies assessing the importance of portal venous pressure and even emergency vascular interventions are needed in order to improve the management of these patients.