Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are inflammatory bowel diseases (IBD). CD is a transmural inflammation that can affect any part of the gastrointestinal tract, with a chronic relapsing clinical course, risk of complications and deterioration of quality of patients’ life [1, 2]. Although the pathogenesis of the disease is not fully explained, its important element is the dysfunction of the mucosal immune system caused by immunological and genetic factors, including mutations of the NOD2 and CARD15 genes, and changes in the intestinal microbiota [1, 2].
The inflammatory process involved in CD is characterized by unbalanced activity of Th1 and Th17 cells with increased production of many pro-inflammatory cytokines including interferon γ (IFN-γ), interleukin 17A (IL-17A), IL-17F, IL-23 and IL-12. Activity of regulatory T cells (Treg) is decreased with a reduction in the production of anti-inflammatory mediators [2, 3].
IFN-γ is one of the key pro-inflammatory cytokines involved in the pathogenesis of CD, exerting immunomodulatory and endothelial effects. It inhibits the activity of Th2 cells, leading to a reduction of the epithelial barrier function and disruption of the vascular barrier [3–5]. IL-12 induces the Th1 polarization and the secretion of IFN-γ, tumor necrosis factor α (TNF-α) and other mediators active in mucosal inflammation. Additionally, IL-12 works with IL-23 to promote the differentiation of Th17 cells [6].
IL-10 is a key anti-inflammatory cytokine influencing the production of pro-inflammatory mediators and other functions of many immunocompetent cells. Defects in IL-10 signaling have been reported to be associated with IBD, especially in children [7]. IL-19 has been found to inhibit the production of pro-inflammatory cytokines by macrophages, Th1 and Th2 cells and has been suggested to have an anti-inflammatory effect in colitis [8].
There is no gold standard in CD diagnosis. This disease is diagnosed on the basis of the clinical presentation, endoscopy with histology, imaging studies including computed tomography and magnetic resonance and laboratory tests [1, 9]. Clinical activity of the disease is assessed using the Crohn’s Disease Activity Index (CDAI) and imaging studies. Laboratory tests performed for CD diagnosis include nonspecific inflammatory markers such as erythrocyte sedimentation rate (ESR), serum C-reactive protein (CRP), and fecal calprotectin [9], and antibodies to Saccharomyces cerevisiae to differentiate CD from UC.
Although the role of many cytokines in the pathogenesis and clinical course of CD is widely researched and well explained, they are not used for diagnostic purposes. Their possible use as markers in the diagnosis of CD must be preceded by an assessment of diagnostic performance. While there is ample evidence about the cytokine networks and serum level profiles in IBD, data on their diagnostic characteristics are scarce.
In our previous paper, we reported an evaluation of the diagnostic performance of serum CRP, IL-6, IL-17A, and IL-23 levels, and their multiplication results in the diagnosis of CD and the assessment of its activity. We found that IL-23 may be a promising candidate marker in the diagnosis of CD, and CRP may be useful in the assessment of disease activity [10]. Continuing this research in the current study, we looked at the diagnostic characteristics of selected pro- and anti-inflammatory cytokines, including IL-12, IFN-γ, IL-10 and IL-19, in the diagnosis and assessment of CD activity.
Material and methods
The study included 49 Caucasian patients with CD, female/male 25/24, aged 36 ±14 years, and 31 healthy controls, also Caucasian, female/male 17/14, aged 52 ±16 years. The patients were diagnosed and treated in the Department of Gastroenterology and Hepatology of the University Hospital, Krakow, Poland. The diagnosis of CD was established by an experienced physician based on the clinical picture and colonoscopy with histology. CD patients were treated at the time of enrollment with glucocorticoids, 6-mercaptopurine, 5-aminosalicylate, infliximab and antibiotics in various combinations. CD patients with coexisting malignancy, endocrine disorders, diabetes, obesity, ischemic heart disease or systemic diseases were not enrolled in the study. In all CD patients the CDAI score was calculated, and they were assigned to the active (exacerbation, CDAI score ≥ 150, 33 patients) and inactive (CDAI score < 150, 16 patients) disease subgroups. The control group consisted of healthy subjects aged 20 to 61 years. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Signed informed consent was obtained from each subject prior to enrolling in the study. The Bioethical Commission of the Jagiellonian University, Krakow, Poland approved the study.
Laboratory tests
The serum was obtained from venous blood samples collected using S-Monovette tubes (Sarstedt, Germany). After clotting and centrifugation, the serum was aliquoted, and samples were stored at –70°C until assayed.
In our previous paper [10], we focused on the diagnostic performance of pro-inflammatory cytokines and CRP, while in this study we aimed to assess the diagnostic characteristics of pro- and anti-inflammatory cytokines involved in the pathogenesis and course of CD. Serum concentrations of IL-6 and CRP were determined using ELISA and immunonephelometry, respectively, as described elsewhere [10]. Serum levels of IL-12, IFN-γ, IL-10 and IL-19 were measured using the following ELISA reagents: Human IL12 (Interleukin 12) ELISA Kit (Miltenyi Biotec, Bergisch Gladbach, Germany), reportable range 3.13–200 pg/ml; Human IFN-gamma ELISA, High Sensitivity (BioVendor, Brno, Czech Republic), reportable range 0.8–25.0 pg/ml; Human IL-10 High Sensitivity ELISA Kit (Diaclone SAS, Cedex, France), reportable range 1.0–50.0 pg/ml and Human IL19 (Interleukin 19) ELISA Kit (Elabscience Houston, USA), reportable range 31.25–2,000 pg/ml. All measurements were performed using the ELx808 spectrophotometer (BioTek R Instruments, Inc., Winooski, USA).
Multiplication results of [IL-6] x [IL-10], [IL-6] x [1L-19], [IL-6] x [IFN-γ], [CRP] x [IL-10], [CRP] x [1L-19], [CRP] x [IFN-γ], [IL-10] x [IL-19], [IL-10] x [IFN-γ], and [IL-19] x [IFN-γ], were calculated using standard concentration units, i.e. mg/l for CRP and pg/ml for interleukins. Serum levels of CRP and IL-6, analyzed elsewhere [10], were used only for these calculations.
Statistical analysis
All variables in the present study, except serum IL-10, showed in the Shapiro-Wilk test a non-Gaussian distribution, so the results are presented as medians and interquartile ranges (IQR, Q1–Q3). Medians were compared using the Kruskal-Wallis ANOVA test and the Dunn test. Spearman’s rank coefficient (R) was used to assess the correlation. P < 0.05 was applied as the significance level.
Diagnostic characteristics in the diagnosis/exclusion of CD and active disease were assessed for serum IL-12, IFN- γ, IL-10, IL-19 levels, and their multiplication results. Relevant cut-off values were established based on the ROC (receiver operating characteristics) curve analysis, and diagnostic sensitivity and specificity, positive and negative predictive values (PPV, NPV), positive and negative likelihood ratios (LR+, LR-) and area under ROC curves (AUC) were calculated.
All statistical analyses were performed using the Statistica 13 software (TIBCO Software, Pao Alto CA, USA).
Results
Diagnosis/exclusion of Crohn’s disease
Serum IL-12, IFN-γ and IL-19 levels were significantly higher in CD patients than in the control group (p < 0.0001–0.007). All multiplication results also differed significantly between CD patients and controls (p < 0.0001–0.017) (Table I).
Table I
Comparison of median (mean for IL-10) concentration of studied cytokines in the serum and the multiplication results in patients with Crohn’s disease (CD) and in the control group
In the diagnosis of CD, the [CRP] x [IL-19] and [IL-6] x [IFN-γ] results had diagnostic specificity of 0.96, PPV of 0.97 and LR+ of 15,0 and 15.5, respectively, while serum IL-19 and the [IL-6] x [IL-12] and IFN-γ] x [IL-12] results had diagnostic sensitivity of 0.9–0.96, NPV of 0.75–0.86 and LR– of 0.09–0.18. The AUC of these markers ranged from 0.776 to 0.807 (Table II). The diagnostic characteristics of the remaining cytokines and the multiplication results in the diagnosis/exclusion of CD were poor.
Table II
Comparison of diagnostic characteristics of the studied cytokines and the multiplication results in the diagnosis of Crohn’s disease
Diagnosis/exclusion of active Crohn’s disease
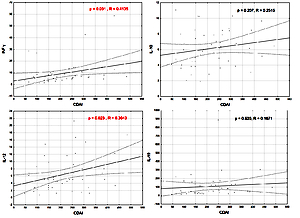
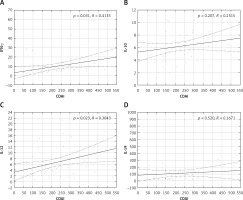
The serum concentrations of the studied cytokines and all their multiplication results were significantly higher in CD patients with active than inactive disease and in patients with inactive disease than in the controls (p < 0.0001–0.023) (Table III). However, only serum IL-12 and IFN-γ significantly correlated (p < 0.05) with the CDAI scores (Figure 1).
Table III
Comparison of median (mean for IL-10) concentration of studied cytokines in the serum and the multiplication results in patients with inactive Crohn’s disease (CD), active CD and in the control group
In the diagnosis of active CD, the [CRP] x [IL-10] and [CRP] x [IL-19] results had a specificity of 0.98, PPV 0.96, LR+ 33.4 and 30.5, and AUC 0.896 and 0.895, respectively. Serum IFN-γ and the [CRP] x [IFN-γ], [CRP] x [IL-12] and [IFN-γ] x [IL-19] results had diagnostic sensitivity of 0.77–0.94, NPV 0.86–0.93, LR– 0.11–0.24 and AUC 0.781-0.904 (Table IV).
Table IV
Comparison of diagnostic characteristics of the studied cytokines and the multiplication results in the diagnosis of active Crohn’s disease
The remaining cytokines and multiplication results performed poorly in differentiating active and inactive CD.
Discussion
CD is an immune-mediated intestinal inflammation driven by activation of Th1 and Th17 cells with increased secretion of their cytokines like IL-12, IL-23, IL-17 and IFN-γ [1, 2], and disease activity is actually the degree of inflammation. Although there is a growing body of evidence of cytokine networks in IBD, the use of these mediators for diagnostic purposes is very limited. The 2016 European Crohn’s and Colitis Organization (ECCO) Consensus recommends checking for chronic inflammatory response with serum CRP, ESR and fecal calprotectin in the diagnosis of CD [9]. Among them, only fecal calprotectin is specifically associated with intestinal inflammation.
There are many cytokines involved in the pathogenesis of CD, but data on their performance in the diagnosis of this disease are scarce. Therefore, the assumption of our study evaluating the diagnostic characteristics of IL-12, IFN-γ, IL-10 and IL-19 was the possibility of using inflammatory mediators involved in the CD pathogenesis as disease markers.
IL-12 is, along with other members of the IL-12 family (IL-2, IL-23, IL-27, IL-35), one of the key mediators of the inflammatory response. IL-12 and IL-23, sharing the p40 subunit, cooperate in a common pathway with IL-23 dominance and play a pivotal role in the induction of mucosal inflammation in the pathogenesis of CD [6, 11, 12]. Increased expression in intestinal samples and higher serum levels of both IL-12 and IL-23 in CD patients have been reported [10, 13], but also no differences in serum IL-12 between CD patients and controls were found [14]. Previously, we found increased serum levels and very good diagnostic performance of serum IL-23 in the diagnosis of CD [10]. In the present study, serum IL-12 and its multiplication results were significantly higher in CD patients than in controls, and in CD patients with active disease (Tables I And II). Additionally, serum IL-12 correlated with the CDAI scores (Figure 1). However, serum IL-12 performed poorly in the diagnosis of CD, but when multiplied by serum levels of IL-6 and IFN-γ, these combined markers performed well in excluding CD (Table II). Serum IL-12 turned out to be useless in the diagnosis of active CD, and only the [CRP] x [IL-12] results performed better (Table IV). These differences in diagnostic characteristics between IL-23 [10] and IL-12 may reflect the predominance of IL-23 in their common pathway.
IFN-γ is another key pro-inγammatory cytokine involved in the pathogenesis of CD. It activates macrophages and lymphocytes, and its additional role is disruption of the vascular barrier and reduction of the epithelial barrier function [2, 5, 15].
Higher serum IFN-γ levels in CD patients than in healthy subjects have been reported in many studies [16–19]. However, IFN-γ is not used as an inflammatory marker in the diagnosis of CD. Measurement of IFN-γ is only for differentiating between CD and intestinal tuberculosis (ITB). T-cell based IFN-γ release assays (IGRAs) provide good diagnostic performance for an accurate diagnosis of ITB [20]. Recently it was reported that serum IFN-γ in combination with IL-6 and fecal calprotectin constituted an index that 6 months after ileocolonic resection for CD had the AUC of 0.90 to predict an early recurrence [21].
In the present study, serum IFN-γ as well as its multiplication results were also significantly higher in CD patients than in controls, and in CD patients with active disease, correlating with CDAI scores (Table I And III, Figure 1). Not serum IFN-γ but the [IL-6] x [IFN-γ] results performed well in diagnosing CD, and [IFN-γ] x [IL-12] results performed well in excluding CD (Table II). Serum IFN-γ and its multiplication by CRP and IL-19 levels performed well in the exclusion of active CD. The AUC > 0.7 also indicated good diagnostic performance of these combined markers (Table IV) [22].
IL-10 as negative regulatory mediator is involved in reducing the effects of T lymphocytes in the intestinal mucosa [23–25]. It is suggested that Th1 and Th17 cells self-limit their inγammatory activity via IL-10 expression [26]. Mutations in IL-10 or its receptor genes have been reported to increase susceptibility to IBD [7, 27].
Elevated IL-10 mRNA expression in the intestinal mucosa was found in patients with both UC and CD [28, 29]. In patients with CD [30, 31] and with active disease [32] higher serum levels of IL-10 than in controls were found. Mitsuyama et al. found elevated serum IL-10 levels during recovery in IBD patients and suggested its use in monitoring disease status [33]. In other studies, serum IL-10 levels in patients with CD did not differ significantly from the controls [14, 34].
In the present study, serum anti-inflammatory IL-10 and its multiplication results were also significantly higher in CD patients than in controls and in CD patients with active disease (Tables I and III).
Only one combined marker, [CRP] x [IL-10], performed very well in the diagnosis of active CD with diagnostic specificity and PPV close to 1.0, LR+ above 30 and AUC > 0.8 (Table IV).
IL-19, belonging to the IL-10 superfamily, functions as an immunomodulatory cytokine [35, 36]. The role of IL-19 in IBD is not fully elucidated and data from in vitro, animal, and human studies are inconsistent. Fujimoto et al. described the possible anti-inflammatory effects of IL-19 in colitis [8]. Defects in IL-19 expression and an impaired response to this cytokine have been reported to possibly contribute to inflammation in active CD [36]. Fonseca-Camarillo et al. found elevated IL-19 and IL-24 gene expression and increased numbers of IL-19- and IL-24-producing cells in patients with active CD compared to healthy controls [37]. Increased IL-19 expression in biopsy specimens and elevated blood levels have been found in patients with active IBD [38].
We also found significantly higher serum IL-19 in CD patients compared to controls and in patients with active CD (Tables I and III). Serum IL-19 performed well in the exclusion of CD (Table II). On the other hand, the [CRP] x [IL-19] results performed well in the diagnosis of CD (Table II) and, with a different cut-off value, in the diagnosis of active CD with the same diagnostic characteristics as [CRP] x [IL-10] (Table IV).
In summary, serum levels of both pro- and anti-inflammatory cytokines studied were significantly higher in CD patients and in patients with active disease although all patients with CD were receiving anti-inflammatory therapy at the time of enrollment and blood collection. These findings may reflect the widely studied and reported cooccurrence of pro-and anti-inflammatory processes in IBD [39–41]. However, only the serum levels of pro-inflammatory cytokines, IFN-γ and IL-12, significantly correlated with CDAI scores, thus reflecting disease activity (Figure 1).
Among the studied cytokines, only IFN-γ and IL-19 showed promising diagnostic characteristics as single markers. Combined markers including [IL-6] x [IFN-γ], [IL-6] x [IL-12], and IFN-γ] x [IL-12] performed well in the diagnosis of CD, and [CRP] x [IL-10], [CRP] x [IFN-γ], [CRP] x [IL-12] and [IFN-γ] x [IL-19] performed well in the diagnosis of active disease. The [CRP] x [IL-19] results turned out to be useful in the diagnosis of both CD and its active form (Tables III and IV). Of note, three of the five combined markers that performed well in the diagnosis of active CD are the results of multiplying the levels of pro- and anti-inflammatory markers.
These findings suggest considering the use of combined inflammatory markers in these settings, as reported by Cerrillo et al. and Kiernan et al. [21, 31]. Billiet et al. found that the ratio of TNF-α/CRP may predict non-response to treatment with infliximab [42]. The search for markers for CD diagnostics continues and goes beyond inflammatory cytokines. Recently, Wędrychowicz et al. found that plasma antimicrobial peptides (elafin, cathelicidin, α-defensins) are associated with the phenotype and location of CD lesions in children [43]. Overall, the analysis of the immune response cannot be overestimated in clinical practice, as demonstrated in the LATE-COVID-Kids Study of the pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) [44].
Completing the CDAI is time-consuming, requires prior complete blood count (CBC) checks, and sometimes requires additional consultations, e.g. by an ophthalmologist. Part of the patient’s response is subjective, which should also be considered a limitation. Regardless of this, we used CDAI as a reference in the assessment of the diagnostic characteristics of the cytokines studied to check the agreement between these tools. For CD monitoring purposes also endoscopy including small-bowel capsule endoscopy and cross-sectional imaging studies utilizing ultrasound, computed tomography, and magnetic resonance are in use [45]. However, these diagnostic tools are resource dependent, not widely available, and have some limitations.
In patients with CD, laboratory tests such as CBC are routinely performed at control visits, so more tests may be ordered. We found that serum IFN-γ, IL-19 and results of multiplication of studied cytokine levels as combined markers can effectively preclude or confirm CD and its active form. Markers with such diagnostic characteristics could be considered a complementary tool and later possibly an alternative to CDAI in the assessment of CD activity.
The important point is that currently all cytokine assays used in our study are approved for research use only. Possible adoption of them as candidate and later recommended markers would require appropriate analytical standardization in accordance with the regulations in laboratory diagnostics. On the other hand, serum CRP and IL-6 measurements are routinely available. It should also be taken into account that the use of combined markers would complicate the diagnostic process and increase its costs.
Due to the small size of the studied groups, our results should be considered preliminary, requiring verification.
In conclusion, this is our latest study evaluating the possibility of extending the panel of inflammatory markers used in CD diagnosis and assessment of its activity. It could make the clinical CD workup faster and more convenient for the patient, easier and cheaper. Our results suggest the need for further evaluation of IFN-γ, IL-19 and selected combined markers, including correlation with the CDAI scores and results of imaging studies.