Introduction
Pancreatitis is an illness distinguished by an inflammatory state, fibrosis, and overall tissue damage, particularly affecting acinar cells of the pancreas. The development and underlying causes are influenced by numerous inflammatory and anti-inflammatory biomarkers released by infiltrated monocytes and macrophages [1]. Acute pancreatitis (AP) is an inflammatory state characterized by a clinical course that can lead to serious local and extrapancreatic organ malfunction and failure. The primary causes of AP, accounting for up to 80% of cases, are gallstones and alcohol consumption [2]. Even though significant advances have been made in the diagnosis and treatment of AP, it still remains a serious and potentially life-threatening disease [3]. The clinical course of AP can range from mild organ malfunction with recovery in a few days to a serious episode that includes organ failure, extreme complications, and a high death rate. Severe acute pancreatitis (SAP) is regarded as an episode of pancreatitis with constant organ failure [4].
The diagnosis of AP can be confirmed if at least 2 of the 3 following criteria are met: abdominal pain typical for AP; pancreatic enzymes, i.e. amylase and lipase higher than 3 times the upper limit of the reference value, and abdominal imaging typical for AP [5].
There is still no confirmed most reliable diagnostic biomarker for AP released from inflamed tissue. The key feature of biochemical diagnosis of AP is related to the measurement of amylase and lipase in the blood. However, lipase is superior to amylase in routine analyses owing to its higher specificity, although with similar sensitivity. Increased activity of lipase in the circulation lasts longer than that of amylase. Moreover, amylase activity is increased in some diseases other than AP, such as abdominal aortic aneurysm, bowel perforation, appendicitis, salivary diseases, ovarian cystoma, rupture of ectopic pregnancy, etc. Moreover, the activity of amylase can be within the reference range in patients with hypertriglyceridemic or alcohol-induced pancreatitis. Also, determination of urine amylase shows no superiority to blood amylase and lipase regarding its diagnostic capability for AP [6].
Pancreatic enzymes can be used only for diagnosis of AP. However, these enzymes are not reliable for the prognosis of AP [6].
Interleukins (ILs) are glycoproteins that are biologically active. They are primarily produced by activated lymphocytes and macrophages. Significant progress in recombinant DNA technology, purification of proteins, and cell-culture techniques has enabled substantial insights into the biochemical and biological features of Ils. These biological properties encompass the ability to stimulate the proliferation and activation of T-lymphocytes, enhance the cytotoxicity of neutrophils, macrophages, and T-lymphocytes, and facilitate the differentiation and growth of B lymphocytes and the bone marrow’s precursor cells that can lead to multiple cell lineages. Ils are also involved in the development of many diseases. It is predicted that IL-based therapy might assume a major role in the treatment of diseases, i.e., cancer, infectious diseases and immunodeficiency syndromes [7].
In AP, the innate immune system recognizes molecular patterns secreted by necrotizing pancreatic cells, which then trigger an adaptive immune response [8]. Cytokines have an important role in the development of AP by initiating an inflammatory response that results in organ malfunction, tissue damage or organ failure in patients with SAP. This inflammatory response includes the recruitment and activation of inflammatory cells, potentially leading to necrosis of the pancreas [9]. The local recruitment and activation of inflammatory cells in AP lead to the production of pro-inflammatory cytokines (e.g., IL-1β, IL-6, IL-8, and tumor necrosis factor-α (TNF-α)) [10], as well as anti-inflammatory mediators including IL-4, IL-10, IL-13, and TGF-β [8]. Previous research demonstrated high production of interferon-γ (IFN-γ), a typical Th1 cytokine, in individuals with AP [11]. Conversely, the role of Th2 cells and their cytokines (IL-4 and IL-5) in the onset of AP and chronic pancreatitis remains a subject of debate. In a Th2-mediated response, IL-4 activates B-cells to secrete immunoglobulin E (IgE) and induces activation of macrophages, which results in an M2 phenotype, whereas IL-5 is crucial in the activation and recruitment of eosinophils [12].
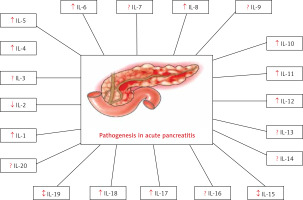
Another approach used in the literature to categorize ILs involves their classification based on their impact on the inflammatory response. This classification results in three distinct groups. The first and largest group comprises inflammatory mediators, encompassing 22 molecules, including IL-1, IL-4, IL-5, IL-6, IL-8, IL-9, IL-13, IL-14, and IL-15. The second group comprises anti-inflammatory biomarkers, consisting of 14 ILs, such as IL-7, IL-10, IL-30, and IL-37. The final group comprises ILs with a dual function, capable of acting as both inflammatory and anti-inflammatory molecules in appropriate circumstances, such as IL-2, IL-3, IL-11, or IL-12 [13]. According to the literature, many ILs have been discovered so far. However, this article represents a summary of the role of ILs in AP (i.e., IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12, IL-13, IL-14, IL-15, IL-16, IL-17, IL-18, IL-19, and IL-20) as presented in Table I and Figure 1. PubMed, Science Direct, and Google Scholar were among the databases used to perform the literature review. The search was conducted until October 26, 2023, and particular keywords such as “Acute pancreatitis,” “Interleukins,” and “Pathogenesis” were used. Clinical research was only conducted if related to English-language literature. No set timeline was established, even though the emphasis was on the current findings. By looking through the reference lists of the chosen papers, additional pertinent publications were found.
Table I
Summary of pathophysiological aspects of interleukins in acute pancreatitis
| Interleukins | Pathophysiological aspects in acute pancreatitis |
|---|---|
| Interleukin-1 |
|
| Interleukin-2 | |
| Interleukin-3 | |
| Interleukin-4 | |
| Interleukin-5 | |
| Interleukin-6 |
|
| Interleukin-7 | |
| Interleukin-8 |
|
| Interleukin-9 | |
| Interleukin-10 |
|
| Interleukin-11 | |
| Interleukin-12 | |
| Interleukin-13 | |
| Interleukin-14 | |
| Interleukin-15 |
|
| Interleukin-16 | |
| Interleukin-17 |
|
| Interleukin-18 |
|
| Interleukin-19 |
|
| Interleukin-20 |
Pathophysiological aspects of interleukins in acute pancreatitis
AP is accompanied by a strong inflammatory response in the pancreas. ILs, as signaling molecules, play a major role in modulating and regulating this inflammatory response. They can either promote inflammation (i.e., pro-inflammatory ILs) or suppress it (i.e., anti-inflammatory ILs), depending on the specific IL involved. During AP, the pancreas can suffer significant tissue damage due to inflammation and the immune response. ILs are responsible for the recruitment and activation of immune cells (i.e., macrophages and neutrophils), which can lead to tissue injury. Understanding the role of specific ILs can help in developing strategies to minimize tissue damage.
Interleukin-1 (IL-1)
IL-1 is viewed as the archetypal pro-inflammatory biomolecule. It exists in two forms, IL-α and IL-1β, and in most research, their biological functions are essentially the same. IL-1 exerts an influence on almost all types of cells, often in conjunction with another pro-inflammatory cytokine called TNF-α. While IL-1 can boost the body’s defense mechanisms and serve as an immune enhancer, it is also an immensely inflammatory cytokine. Striking a balance between achieving clinical benefits and avoiding undesirable side effects in humans is a precarious task. Conversely, substances that decrease the activity or production of IL-1 are believed to have a substantial impact on clinical studies. The processes involved in the synthesis, processing, secretion, and effects of IL-1, particularly IL-1β, are meticulously regulated. A noteworthy aspect of cytokine biology is the presence of a naturally occurring antagonist known as IL-1 receptor antagonist (IL-1RA). IL-1RA shares structural similarities with IL-1β but lacks agonistic activity. It is employed in clinical trials to mitigate disease severity. Moreover, the control of IL-1’s activity extends to factors such as limited numbers of surface receptors, soluble receptors in circulation, and a surface “decoy” receptor that down-regulates responses to IL-1β [14].
Genetic variations found within the IL-1RA gene are the most influential genetic factors that determine the levels of IL-1RA in the bloodstream. Consequently, these genetic variants can be employed as genetic tools in Mendelian randomization analyses [15]. Higher genetically predicted IL-1RA values were linked to a reduced likelihood of experiencing AP and chronic pancreatitis, as well as developing pancreatic cancer. In a meta-analysis involving data from the FinnGen and UK Biobank, the combined odds ratio was 0.87 (with a 95% confidence interval of 0.77–0.97, and a significance level of p = 0.003) for AP. It suggested that IL-1RA may play a protective role in 3 significant diseases of the pancreas, suggesting the potential therapeutic applications of IL-1RA in the treatment [16].
IL-1 is a cytokine known for its pro-inflammatory properties, and it has been observed to be secreted during AP. Previous animal research conducted on moderate/severe cases of AP indicated that blocking this potent biomolecule was linked to reduced damage to the pancreas and significant improvements in survival rates. However, the precise properties of IL-1 and whether its receptors’ activation is necessary for the pancreatitis onset and/or progression remain uncertain. In cases where the IL-1 receptor’s genetic absence or its pharmacological blockade was employed, there was a notable decrease in enzymes released by the pancreas, as well as diminished pancreatic inflammation, edema and necrosis. Furthermore, the level of serum IL-6, which serves as a marker of the severity of inflammation, was substantially reduced in both groups. It was suggested that while the IL-1 receptor’s activation might not be a prerequisite for the onset/progression of AP, it appears to be essential for the maximum amplification of pancreatic injury and the accompanying inflammatory response [17].
Abundant quantities of IL-1β are generated during AP, and it is thought to have a major influence in recognizing the significance of AP and the extent of damage to tissue of the pancreas. Fink et al. [18] explored the production of IL-1β and other genes within the IL-1 family within the pancreas during sterile AP. It revealed that there was consistent expression of IL-1R1, IL-1RA, IL-1R2 and caspase-1 (also known as interleukin-1β converting enzyme (ICE)) genes in the pancreas, although IL-1β expression was absent at baseline. As pancreatitis progressed, the mRNA levels for IL-1RA, IL-1β and ICE increased in tandem with the AP severity, while the mRNA levels for both receptors remained unchanged. Furthermore, the levels of IL-1RA proteins and IL-1β within the pancreas and in the bloodstream increased as AP progressed, with values in tissue consistently surpassing those in the serum. It illustrates that sterile AP without exposure to endotoxins triggers upregulation of specific genes within the IL-1 family, including substantial synthesis of IL-1β and its receptor antagonist within the tissue of the pancreas. Such alterations are regarded as markers of AP severity and were not dependent on the specific experimental model used [18].
The progression over time and the connection between the levels of cytokines in the circulation, inflammation within the pancreas, and organ dysfunction during AP are not well understood. It indicated that there was a release of mediators within the pancreas, possibly leading to a diminishing balance between IL-1β and IL-1RA in severe cases. Subsequently, there was a systemic presence of both pro-inflammatory and anti-inflammatory biomolecules. The observed scheme of mediators at both systemic and local levels in severe AP suggests that systemic activation of lymphocytes, possibly provoked by the local release of these biomolecules, may initiate the development of distant organ complications in SAP [19].
Some experimental research suggested that inhibiting ICE can significantly improve the overall survival and severity in cases of severe experimental AP. The use of the ICE inhibitor notably decreased the extent of the necrosis of acinar cells, which was responsible for key damage to the pancreatic tissue. Conversely, apoptosis occurred mainly during the later stages of the disease and was closely linked to the formation of tubular complexes, with both phenomena remaining unaffected by the treatment. While the pancreatic mRNA expression of IL-1β increased significantly in both untreated and treated animals, the active expression of IL-1β protein and the consequent neutrophil infiltration into the tissue were substantially reduced in the ICE inhibitor treated group. As apoptotic acinar cell death and the formation of tubular complexes intensified, the mRNA and protein expression of TNF-α increased, with levels being lower in the ICE inhibitor treated rats. In the summary, it was suggested that the activation of caspase-1/ICE plays a pivotal role in the aggravation of acinar cell necrosis and death in SAP. In this context, IL-1β-related neutrophil infiltration appears to be a critical stage in exacerbating the destruction of the acinar cells. On the other hand, the apoptosis of the acinar cells favors the ductal transformation and operates independently of this process, although TNF-α might influence it [20].
The role of IL-1β in the development of AP was shown through significant reductions in pancreatic damage and notable improvements in survival when its actions are blocked. While it is known that the pancreas is a key source of IL-1β during AP, the specific cells responsible for this production have not been identified. To explore this further, it was hypothesized that infiltrating leukocytes might be a significant contributor, and examined the intrapancreatic IL-1β synthesis after manipulating specific leukocytes in vivo before inducing pancreatitis. It was found that leukocytes (i.e., macrophages and neutrophils) that infiltrate the pancreas were the main culprits for the IL-1β production within the pancreas during AP. Elimination of the leukocytes and their products, including IL-1β, favors amelioration of the severity of pancreatic damage [21].
Numerous epidemiological investigations have confirmed the connection between variations in IL genes and the occurrence of AP across various populations. Nevertheless, there is a scarcity of studies conducted within Asian ethnic communities. The potential associations between polymorphisms in inflammatory cytokine genes and AP were investigated as an initial study within a Korean ethnic population. It was observed that certain genotypes of IL1RA−1129T>C (rs4251961) could be linked to a notable increase in the risk of developing AP among individuals of Korean ethnicity [22].
Fulminant AP is a complex disease with multiple factors contributing to its onset, resulting in the activation of various proinflammatory mediators. Since IL-1 plays a crucial early role in the acute inflammatory process, researchers investigated the potential therapeutic benefits of using an IL-1RA in experimental AP. Norman et al. [23] assessed the effectiveness of blocking early cytokine activity to further elucidate the role of inflammatory cytokines in the development of AP. The use of moderate (10 mg/kg) and high (100 mg/kg) IL-1RA doses significantly reduced TNF-α, IL-6 and lipase values and pancreatic wet weight. When IL-1RA was given before induction, it also significantly lowered serum amylase activity, although not when given after induction. Additionally, histological assessments showed a notable decrease in inflammatory cell infiltration, edema and necrosis in the pancreas, in all animals that were administered high or moderate IL-1RA doses. However, low IL-1RA doses (1.0 mg/kg) had modest effects when administered prior to the induction but showed no effects when administered after. Elevated proinflammatory cytokines, such as TNF-α and IL-6, are commonly observed during experimental AP and are closely linked to the extent of local pancreatic damage. Blocking the inflammatory cascade at the IL-1 receptor level, either before or shortly after pancreatitis induction, substantially mitigated the increase in these cytokines and was associated with reduced pancreatitis severity and decreased intrinsic pancreatic injury [23].
Interleukin-2 (IL-2)
IL-2 is a multifaceted cytokine. Comprehending the signaling pathways that enable IL-2 to govern the differentiation and equilibrium of both pro-inflammatory and anti-inflammatory T cells is crucial for unravelling the precise mechanisms of immune regulation. The IL-2 receptor is connected with Janus kinases (JAK) and triggers the activation of STAT5 (signal transducers and activators of transcription) transcription factors. Nevertheless, IL-2’s role extends beyond merely regulating transcriptional programs. It serves as a pivotal controller of metabolic processes in T cells. The advancement of comprehensive phosphoproteomic approaches has broadened the comprehension of IL-2 signaling, unveiling the array of phosphoproteins that IL-2 might potentially influence in T cells [24].
The crucial role of the cytokine IL-2 in maintaining normal immune function and defending against infections following burns and trauma has long been acknowledged. However, insufficient attention has been given to its potential significance in AP, a condition also connected with a high mortality rate due to sepsis. The potential involvement of IL-2 in mice with diet-induced AP was investigated. The results revealed a significant decrease in IL-2 secretion on day 3 (32% reduction; statistically significant at p < 0.05) and day 10 (48% reduction; statistically significant at p < 0.005). Additionally, the administration of intraperitoneal lipopolysaccharide on day 10 led to decreased IL-2 production 4 h later, accompanied by a 90% mortality rate in AP-afflicted animals. In vivo, treatment with recombinant IL-2 not only ameliorated in vitro IL-2 secretion, but also reduced mortality induced by lipopolysaccharide. It suggested that diet-induced AP in mice was linked to compromised immune function and heightened vulnerability to sepsis, making it a valuable model for investigating immunomodulation in the context of AP [25].
Another study was conducted involving measurements of various cytokines including proinflammatory ones such as IL-1β, IL-6, and IL-8, as well as the anti-inflammatory mediator IL-10, IL-1RA, and the soluble IL-2 receptor (sIL-2R). It examined how these measurements correlated with complications affecting both specific organs and the systemic condition in individuals with AP. As a result, it was found that patients who experienced distant organ failure exhibited significant elevation of sIL-2R, IL-10 and IL-6 levels [19].
Interleukins 4 and 5 (IL-4 and IL-5)
IL-4 is an 18 kDa cytokine that can be secreted and potentially glycosylated. It possesses anti-inflammatory and immunomodulatory properties, and it has a diverse range of functions, some of which overlap with those of IL-13. IL-4 is primarily produced by a specific subgroup of activated T-cells known as Th2 CD4(+) helper cells. It plays a role in promoting the growth and differentiation of activated B-cells, as well as in enhancing the expression of class II MHC antigens and low-affinity IgE receptors in resting B-cells [26]. Likewise, Zhang et al. [27] aimed to examine how IL-4 influences the changes in the expression of regulators that control complement activation in the pancreas and the occurrence of pancreatic necrosis in experimental SAP. Complement activation regulators are assumed to be included in the onset of pancreatic inflammation. The reduction in the expression of these regulators might contribute to the occurrence of pancreatic necrosis. Treatment with IL-4 could potentially mitigate the worsening of SAP by boosting the levels of CD59 and decay accelerating factor (DAF) in the pancreas while reducing pancreatic necrosis. Additionally, it appears that CD59 and DAF may have a significant role in regulating complement activation regulators during SAP [27].
AP is a relatively common condition, and for most patients, it follows a benign course. However, in about 20% of cases, the disease takes a more severe and potentially fatal course, with mortality rates ranging from 40% to 50%. The assessment of the disease severity is currently based on protocols that encompass laboratory, clinical and radiographic diagnostic methods, along with scoring systems such as Acute Physiology and Chronic Health Evaluation (APACHE II), the CT index, the CT necrosis score and the Ranson score. T lymphocytes are pivotal in the immunopathogenesis of AP, and recent research has shed light on the role of Th2 cells and their effector cytokines, namely IL-4 and IL-5. IL-5 is a cytokine involved in the recruitment, multiplication, maturation, and activation of eosinophils. It also enhances the adhesion and movement of eosinophils on periostin. Recent findings suggest that IL-5 plays a role in the buildup of pancreatic fibrosis. Studies on mice lacking IL-5 show reduced eosinophil infiltration and decreased collagen production following cerulein injections compared to mice with an intact IL-5 gene. Furthermore, IL-5 can stimulate the growth and development of B cells and can sensitize mast cells, increasing their production of cytokines that support tumor growth and fibrosis, including IL-5, IL-13, TNF-α, macrophage inflammatory protein-1α (MIP-1α) and granulocyte-macrophage colony-stimulating factor (GM-CSF). Additionally, in the presence of pancreatic stroma, IL-5 favors the synthesis of collagen and may trigger the differentiation of M2 macrophages. While the IL-5 receptor (IL-5Rα) has been well recognized in the context of immune cell signaling, its expression in other types of tissues has not been extensively studied [28].
The potential clinical utility of IL-4 and IL-5 for predicting the onset of SAP with necrosis and systemic complications, such as systemic inflammatory response syndrome (SIRS), was examined. It was observed that IL-4 and IL-5 levels showed a statistically significant increase on the second day of hospitalization, reaching their peak levels on the third day. In patients with SAP complicated by necrosis and/or sepsis, these levels continued to increase throughout the seventh day. Furthermore, it was found that the levels of IL-5 and IL-4 could serve as early indicators of the severity of AP, as well as early markers of sepsis and overall prognosis, given their significant statistical correlation with outcomes, the presence of SIRS and the Ranson score [29].
Interleukin-6 (IL-6)
The two receptor chains and molecules that send signals downstream make up the interleukin-6 (IL-6) receptor signaling system [30]. The IL-6 receptor, also known as IL-6R or sIL-6R, is the chain that binds to IL-6 and comes in two different sizes: an 80 kDa transmembrane form and a 50–55 kDa soluble form. The 130 kDa protein known as gp130 is an example of the signal-transducing chain. The Trp-Ser-X-Trp-Ser motif is shared by both of these proteins, which are members of the cytokine receptor family [31, 32]. The cytoplasmic part exists in human serum but is lacking in sIL-6R. In cells that express gp130, the complex formed when IL-6 binds to sIL-6R initiates the IL-6 signal [33]. The broad presence of gp130 in these cells accounts for the vast spectrum of effects that IL-6 has on various cells [34]. When IL-6 binds to IL-6R, this complex causes the pairing of gp130 molecules, which then triggers a series of downstream signals [35].
IL-6 is a soluble mediator with several effects on the immunological response, hematopoiesis, and inflammation. IL-6’s many uses were first given distinctive names based on their unique biological actions. Because it can encourage the development of active B cells into antibody-producing cells, it was for example given the name B-cell stimulatory factor 2 [36]. Since it causes acute phase protein production in hepatocytes, it was given the name hepatocyte-stimulating factor. The fact that the hybrid cells created by fusing plasma and myeloma cells may develop faster is reflected in the name of the growth factor, hybridoma growth factor. Due to its comparable antiviral properties to IFNs, it was also referred to as IFN-β2 [37].
In several studies, the prognostic significance of IL-6, IL-8, IL-10, and CRP for predicting the outcomes of AP has been investigated. However, there is a large amount of variation in the findings between studies. Within 48 h of the beginning of symptoms, IL-6 levels of 28.9 pg/ml showed the strongest predictive value for identifying the development of AP [38]. IL-6 has been linked to a number of exocrine pancreatic disorders that are extremely onerous, including pancreatic cancer, chronic pancreatitis and AP. However, the specific function of IL-6 in various illnesses is still complex and poorly understood. The Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway is activated by IL-6, which worsens acute and chronic pancreatitis and promotes the onset and/or progression of pancreatic cancer, according to a growing body of research [39].
Recombination signal binding protein for immunoglobulin kappa J region-like protein, or Rbpjl, expression may have been decreased in AP, according to a microarray data analysis. Since IL-6, STAT3, and Arid5a (AT-Rich Interaction Domain 5A) are all known to have a role in the immune system’s ability to control AP development, bioinformatics predictions suggested that these three molecules may interact. Determining how the Arid5a/IL-6/STAT3 axis influences Rbpjl’s regulatory function in AP inflammation was the focus of a separate investigation. In pancreatic tissues of AP mice and acinar cells generated by LPS, Rbpjl was shown to be downregulated. Increased cell viability, a reduction in inflammation and reactive oxygen species (ROS) formation brought on by LPL, and a reduction in AP-induced damage were all outcomes of the re-introduction of Rbpjl. In a mechanism that inhibits activation of the IL-6/STAT3 signaling pathway, Rbpjl was able to bind to the Arid5a promoter region and down-regulate the production of this gene. Rbpjl also reduced the activation of IL-6 and STAT3 that is reliant on Arid5a, which reduced inflammation of the pancreatic acinar cells. Arid5a expression was suppressed and the IL-6/STAT3 pathway was deactivated by Rbpjl, which, in turn, helped to lessen the effects of AP [40].
However, in around 20% of patients, AP can advance to SAP, which is defined by systemic inflammation and/or pancreatic necrosis. AP normally progresses in a self-limiting course of localized inflammation. For suitable intensive therapy to be started and mortality to be decreased, SAP must be identified early. The highest IL-6 levels on both days were seen in patients with SAP. IL-6 exhibited positive correlations with markers of inflammation (such as CRP, white blood cell and neutrophil counts, and procalcitonin (PCT)), renal injury (such as neutrophil gelatinase-associated lipocalin and kidney injury molecule-1) and endothelial dysfunction (such as soluble fms-like tyrosine kinase-1 and angiopoietin-2). The areas under the receiver operating curve (ROC) (AUC) for IL-6 levels at entry ranged from 0.75 to 0.78, which were equivalent to multi-variable prognosis ratings and were strong predictors of SAP, vital organ failure, the requirement for intensive care, or mortality. The greater clinical use of this important biomarker is made possible by the use of a fully automated assay that enables quick and accurate detection of blood IL-6 [41].
In the study by Li et al. [42] it was examined whether IL-6 is more accurate than CRP at foretelling fatal outcomes, including severe AP and infected pancreatic necrosis (IPN). A strong positive connection was discovered between the severity of AP and blood levels of IL-6 and CRP. When compared to IL-6, CRP had superior predictive ability for organ failure and pancreatic necrosis. Although IL-6 outperformed CRP in terms of accuracy in predicting mortality and IPN, CRP still had a lower AUC than IL-6. AUC 0.79 vs. 0.72 for predicting SAP showed that the combination of SIRS and CRP exhibited higher accuracy than the combination of SIRS and IL-6. IL-6 was shown to be exhibit higher accuracy than CRP at predicting IPN and mortality in individuals with AP [42].
The role of microRNAs (miRNAs/miRs) in combination with inflammatory markers in determining the severity of AP was investigated. Patients with moderate AP (MAP), SAP, and recurring AP (RAP) had their serum levels of pro-inflammatory mediators, such as IL-1, TNF-α, IL-6, IL-8, and IL-10, as well as of miRNAs, such as Homo sapiens (hsa) miR 548d 5p, hsa miR 126 5p, and hsa miR 130b 5p, tested. According to the findings, MAP and RAP can be distinguished from SAP by having high expression levels of IL-10, TNF-α, hsa miR 126 5p, hsa miR 548d 5p, and hsa miR 130b 5p. According to these results, serum IL-6 and hsa miR 126 5p detection in combination may be reliable for AP prediction [43].
Interleukin-8 (IL-8)
IL-8 belongs to the C-X-C chemokine family, which plays a role in recruiting various immune cells such as polymorphonuclear neutrophils, lymphocytes, eosinophils and basophils and to sites of inflammation. IL-8 serves as both an activator and a chemoattractant for neutrophils. In rats, the growth-regulated gene product/cytokine-induced neutrophil chemoattractant (GRO/CINC)-1 is the equivalent of IL-8 in humans. GRO/CINC-1 comprises 72 amino acids, which are structurally similar to human peptides associated with melanoma growth stimulation. This similarity suggests that GRO/CINC-1 shares both functional and structural homology with human IL-8. GRO/CINC-1, or IL-8, is expressed in animal models such as gastric ulcers, ischemia-reperfused liver injuries, and AP. In humans, elevated serum levels of IL-8 have been reported as an early indicator of AP severity and complications. In experimental AP, the use of an anti-IL-8 neutralizing antibody has been shown to inhibit cytokine responses and prevent acute lung injury. Although recent findings have indicated the presence of IL-8 mRNA in human chronic pancreatitis, the specific role of IL-8 in the development of this disease remains uncertain. Obstructive pancreatitis is a distinct form of pancreatitis caused by blockage of the main pancreatic duct, often due to factors such as tumors. This type of pancreatitis differs from chronic pancreatitis, which is typically associated with alcohol abuse or gallstones. However, tissue from obstructive pancreatitis was frequently available during pancreatic surgery. Analyzing IL-8 expression in obstructive pancreatitis could potentially enable valuable insights into the role of IL-8 in the pathogenesis of chronic pancreatitis or related fibrosis [44].
White blood cells, particularly neutrophils, play a significant role in the development of complicated pancreatitis. Elevated levels of neutrophil elastase, a marker of activation of neutrophils, have been observed in patients with severe AP. More recently, IL-8 has emerged as a new biomarker known to activate neutrophils. Patients with complicated pancreatitis exhibited significantly higher levels of both neutrophil elastase and IL-8 compared to uncomplicated cases. A positive correlation was observed between IL-8 and neutrophil elastase, particularly in the lower concentration range of IL-8. During follow-up, initially, higher IL-8 levels decreased in parallel with clinical amelioration [45].
The presence of infection within pancreatic necrosis significantly influences the clinical trajectory, management strategies, and ultimate outcomes in cases of AP. Currently, the only method to achieve an early and precise diagnosis of infected necrosis is through guided fine needle aspiration. PCT, a propeptide consisting of 116 amino acids related to calcitonin, and IL-8, a potent cytokine that activates neutrophils, serve as markers indicating severe inflammation and sepsis. These markers are detected in elevated concentrations in cases of infected necrosis, as well as in systemic complications associated with AP. The levels of PCT in the bloodstream demonstrate the close link with the presence of infected necrosis. Monitoring serum PCT levels holds promise as a novel non-invasive method for accurately predicting the presence of infected necrosis and for selecting patients who continue to experience septic complications following surgical debridement [46].
Early identification of patients at risk for SAP is of utmost importance. Various serum markers have been examined for their potential to predict disease severity, among them IL-8 and IL-6. However, the accuracy of these markers in predicting SAP has shown significant variability in different studies. For IL-6, the AUC values were 0.75, 0.88, and 0.85 for days 1, 2, and 3, respectively. For IL-8, the AUC values were 0.73 on day 1 and 0.91 on day 2. On day 1, the diagnostic odds ratio for IL-6 was higher than that for IL-8 [47].
Numerous studies have explored the potential links between the rs4073 polymorphism of IL-8 and AP. However, the findings have been inconclusive in terms of providing a more precise assessment of the association between the IL-8 rs4073 polymorphism and AP. This meta-analysis provides suggestive evidence that individuals carrying the A risk allele of the IL-8 rs4073 polymorphism may have a heightened susceptibility to AP [48].
Innate immune response-related inflammatory factors play pivotal roles in the development of AP. Anilir et al. [49] assessed the connection between IL-8 gene polymorphism and the types as well as severity of AP. They identified a significantly higher occurrence of the IL-8 AA genotype among patients with acute biliary pancreatitis, particularly in those with alanine transaminase (ALT) levels exceeding the median range. Furthermore, homozygote alleles were notably more prevalent among patients with acute biliary pancreatitis who exhibited amylase levels above the median range. It sheds light on the frequency of IL-8 polymorphism in the context of AP, offering valuable insights and supporting the role of IL-8 in diagnostic assessments [49].
AP is a potentially life-threatening condition, particularly when it takes on a severe form, accounting for approximately 20% of cases of hospitalized AP patients. The role of β2 microglobulin (B2M), IL-8 and IL-6 in evaluating the severity of pancreatitis was assessed. It led to several significant findings, including a substantial increase in the total leukocyte count in the SAP group compared to the MAP group. Additionally, there was a highly significant elevation in baseline B2M, IL-8 and IL-6 levels in the SAP group compared to the MAP group. On the third day, B2M, IL-8 and IL-6 levels remained significantly higher in the SAP group in comparison with the MAP counterparts. Lastly, the SAP group exhibited a notably higher incidence of complications and mortality rates compared to the MAP group. These findings underscore the importance of inflammatory markers, particularly ILs, as valuable tools for predicting and monitoring patients with AP [50].
Another study investigated whether IL-8 and hepatocyte growth factor (HGF) can serve as predictors of the onset of SAP in individuals who do not initially exhibit organ dysfunction (OD), and whether these markers can differentiate between transient OD and persistent OD in patients presenting with OD. In patients with AP who might not have OD at the time of presentation, the levels of circulating IL-8 or HGF may be indicative of the likelihood of developing SAP. On the other hand, in patients who present with OD, the level of IL-8 may be used to distinguish between those with transient OD and those with persistent OD [51].
Excessive activation of white blood cells has been suggested as a critical mechanism triggering the onset of AP. In severe cases, IL-8 levels began to rise from day 0, while in mild cases, they increased starting from day 1, reaching a stable level between days 2 and 5. Significant differences in IL-8 levels were observed on days 0 and 1. These discoveries offer fresh insights into the significance of factors involved in keeping the balance between inflammation and anti-inflammation in AP. It appears that these molecules can serve as valuable early indicators of disease severity [52].
Interleukin-10 (IL-10)
IL-10 was initially identified as a cytokine produced by T helper 2 cells, but it was later realized that practically all leukocytes produce this cytokine, which has a wider distribution than only certain T cell subsets [53]. T helper cells, macrophages, monocytes and dendritic cells (DC) are important in vivo donors of IL-10. Nevertheless, a wide range of immune effector cell types, such as B cells, cytotoxic T cells, mast cells, natural killer cells, as well as granulocytes such as neutrophils and eosinophils, can produce IL-10 under appropriate circumstances [54–56]. The capacity to release IL-10 in response to infection, tissue injury, and even within tumor cells is also seen in non-immune effector types including keratinocytes and epithelial cells [57].
One of the key factors in restricting the host’s immune response to infections is the cytokine IL-10, which is well known for its potent anti-inflammatory characteristics. The natural tissue balance and prevention of host injury are both maintained. When IL-10 is misregulated, there is a greater tendency for infections to cause immunopathology, and there is also a greater chance of developing a number of autoimmune illnesses. The course of the disease and the cessation of the host’s inflammatory response can thus be understood only by having a thorough grasp of how IL-10 gene expression is controlled. Many processes, such as signal transduction, promoter architecture, epigenetics and post-transcriptional regulation, control the expression of the IL-10 gene in distinct immune effector cells [58].
The heterodimeric IL-10 receptor, which is made up of IL-10R1 and IL-10R2, interacts with IL-10 to support its immunosuppressive function. Macrophages and monocytes seem to be the primary targets for IL-10, despite the fact that the expression of the IL-10 receptor complex varies among a variety of cell types. When the receptor is activated, JAK/STAT signaling begins, significantly altering the expression pattern of genes responsible for immune control [59]. Because of this, the secretion of pro-inflammatory biomarkers is inhibited, antigen presentation and phagocytosis are decreased, and the inhibitory, tolerative, and scavenger activities of mentioned cells are simultaneously improved. Furthermore, IL-10 can exert an influence directly or indirectly on T lymphocytes, inhibiting the development of certain cell types [60], dampening cellular responses [61] and boosting the function of regulatory T cells [62].
A potentially fatal condition called pancreatic encephalopathy can arise when the blood-brain barrier (BBB) is compromised by SAP. A well-known cytokine with strong anti-inflammatory and immunoregulatory effects is IL-10. Uncertainty persists regarding the precise ways in which IL-10 protects the BBB in SAP. Through the STAT3 pathway, IL-10 was found to reduce brain microvascular endothelial cells’ (BMECs’) apoptosis and to mitigate the BMECs’ loss of claudin-5 expression in in vivo studies using SAP rat models. Inflammation increases BBB permeability. In experiments conducted in vitro, IL-10 increased BBB integrity against TNF-α by preventing tight junction structures from being disrupted, reducing BMEC death through the STAT3 pathway, and compensating for BMECs’ decreased expression of claudin-5. IL-10 enhances the BBB’s capabilities in SAP by inhibiting the down-regulation of claudin-5 expression, maintaining tight junctions, and acting on BMECs in a manner that inhibits apoptosis via the STAT3 pathway [63].
The function of IL-10 in controlling the development of pancreatic fibrosis and the regeneration phase in AP was studied. It is well known that IL-10 inhibits inflammatory cells’ stimulation-induced production of transforming growth factor-β. In this study, AP caused by repeated administration of cerulein was inflicted upon IL-10 knockout (KO) mice and their C57BL/6 control counterparts. Immunohistochemistry was used to find active stellate cells, and tritiated thymidine was used to mark intrapancreatic cells in the S phase. In comparison to the control group, IL-10 KO mice following recurrent AP displayed more severe histological lesions and fibrosis (higher intrapancreatic collagen content). In IL-10 KO animals, there was a considerably higher amount of TGF-β (TGF-1) in the plasma, intrapancreatic transcription, expression by interstitial and ductal cells, as well as the quantity of activated stellate cells. Furthermore, acinar cells were less abundant in the S phase in IL-10 KO animals, but pseudotubular cells showed the reverse trend. The results indicate that endogenous IL-10 is essential for regulating the regeneration phase and preventing severe fibrosis and glandular atrophy brought on by recurrent AP episodes in mice [64].
The efficacy of IL-10 when administered before the beginning of experimental AP has been demonstrated in prior studies. Rongione et al. [65] investigated whether or not IL-10, a cytokine that limits macrophage production of inflammatory biomolecules, may change the severity of AP whether administered before or after the induction of the condition. According to the results, serum levels of amylase, TNF-mRNA, and TNF-α protein significantly decreased when IL-10 was given either before or after the onset of AP. Pancreatic edema and the histopathological score were also diminished. Serum TNF-α levels were not detectable. According to these findings, IL-10 reduced the severity of experimental AP whether it was given before or after the condition started [65].
Serum IL-10 levels rose under normal circumstances and showed a link with the development of pancreatitis, peaking 8 h after introduction. The pancreas, liver, and lungs all had IL-10 and TNF-α messengers, which all showed comparable patterns. The severity of pancreatitis and the accompanying lung damage increased considerably when endogenous IL-10 was neutralized. This was followed by a 75% rise in blood TNF-α protein levels and increased TNF-α messenger expression in the liver (+43%), pancreas (+33%), and lungs (+29%). The systemic production of IL-10 coincided with the progression of the illness in this AP model that was not deadly. Both TNF-α and the anti-inflammatory response were released simultaneously, and both cytokines were generated by various bodily systems. Endogenous IL-10 functioned to regulate TNF-α production and provided protection by reducing the local and systemic effects of AP [66].
It is assumed that AP develops mostly as a result of inflammatory processes. The production of proinflammatory cytokines by macrophages and/or monocytes has been found to be significantly inhibited by IL-10. Utilizing a mouse model of acute necrotizing pancreatitis (ANP), another study looked at the potential protective benefits of IL-10. The release of systemic amylase and lipase, which are indicators of pancreatitis, peaked 9 h after the initial cerulein injection. IL-10 treatment, however, dramatically lowered this peak. Histological findings of the mice showed that both groups of mice had pancreatic edema and inflammation, but the IL-10-treated animals displayed a striking decrease in pancreatic necrosis. TNF levels in the bloodstream were not even detectable in this mouse. The researchers also noted that the expression of TNF messenger RNA was much lower in the IL-10-treated group compared to the control group with AP when they examined the pancreatic tissues during the period of the greatest morphological abnormalities. Overall, it was found that IL-10 significantly reduced the severity of experimental AP by largely preventing the onset of acinar necrosis. The capacity of IL-10 to inhibit local TNF production may be, at least in part, responsible for the protective properties [67].
Despite the extensive research carried out, AP is currently without a particular therapy. Pirfenidone, the Food and Drug Administration (FDA)-approved anti-inflammatory and antifibrotic medication used to treat idiopathic pulmonary fibrosis (IPF), was tested to determine whether it may reduce AP’s systemic and localized damage. Palathingal Bava et al. [68] evaluated this drug’s efficacy in this regard. The results showed that therapeutic administration of pirfenidone, even when started at the height of injury, substantially lessens the degree of local and systemic damage and inflammation in a variety of AP models. Pirfenidone was shown in laboratory trials to reduce the production of cytokines from acinar cells, and macrophages interfere with the communication between these two cell types. Prior to the appearance of detectable histological alterations, pirfenidone therapy increased the release of IL-10 from macrophages. It also had an impact on the immunological properties of inflammatory cells by lowering inflammatory cytokine levels. Research was carried out utilizing IL-10 mutant mice, macrophage depletion, and antibody-mediated IL-10 depletion to corroborate the mechanism of action. These all showed how crucial IL-10 and macrophages are for pirfenidone’s therapeutic actions. It would be possible to launch a clinical trial right away to examine the effectiveness of pirfenidone in moderate to severe AP patients given that the drug has already received approval from the FDA for IPF [68].
Recent research has identified IL-10 as an anti-inflammatory cytokine that prevents monocytes and/or macrophages from producing proinflammatory cytokines and from releasing toxic oxygen radicals. According to studies, cellular necrosis suppression is the main mechanism by which IL-10 therapy lessens the severity of experimental pancreatitis. On the first day of the disease in patients with AP, blood IL-10 levels were shown to be elevated, then to gradually fall over the following days. Patients with a milder type of AP had considerably higher values of IL-10 than those with a severe form on the first day of the condition. However, no significant difference between the two groups in the days that followed was observed. Patients with MAP had a considerable rise in IL-10 on the first day of illness, as opposed to those with a severe form of the condition. Low levels of IL-10 were found in the blood of people with SAP, which may point to defective down-regulation of the immune-inflammatory response in such patients [69].
Interleukin-11 (IL-11)
IL-11, a cytokine originating from stromal cells, has a broad range of effects in both hematopoietic and non-hematopoietic systems. IL-11 promotes the growth of specific plasmacytoma and hybridoma cells, collaborates with IL-3 to reduce the dormant phase in early progenitor cells, supports the formation and maturation of megakaryocyte colonies, and serves as a growth factor in megakaryoblastic cell lines. Additionally, IL-11 stimulates the production of red blood cells, enhances immune responses specific to antigens, triggers the synthesis of acute phase proteins, inhibits the activity of lipoprotein lipase and the differentiation of adipocytes, and facilitates neuronal development. When recombinant human IL-11 (rhIL-11) is administered to mice, it leads to an increase in neutrophils and platelets. The human IL-11 gene is situated at 19q13.3-13.4, encoding a protein consisting of 199 amino acids with a molecular weight of 23 kDa and lacking N-glycosylation. Its receptor and signaling pathways partly overlap with those of IL-6. A more thorough exploration of its role in normal and pathological conditions is imperative to precisely define its functions and potential clinical applications [70].
IL-11, belonging to the IL-6 family of cytokines, is associated with several fibro-inflammatory diseases. IL-11 binds to its specific α receptor, IL-11RA, which is highly expressed in stromal cells such as fibroblasts and hematopoietic stem cells (HSCs). Upon binding, IL-11 signals through the gp130 receptor, leading to the activation of ERK and, to a lesser extent and for a short duration, STAT3. Previous research has indicated that serum IL-11 levels are increased in patients with SAP. While IL-11 is known to play a crucial role in inflammation and fibrosis, its specific role in the pancreas has remained uncertain. In cases of pancreatitis, which is characterized by inflammation, fibrosis and organ malfunction, the transformation of pancreatic stellate cells (PSCs) into myofibroblasts is a key process. It demonstrated that when PSCs, which express IL-11RA in the pancreas, are stimulated with IL-11, it leads to transient phosphorylation of STAT3, sustained activation of ERK, and activation of PSCs. In contrast, stimulation of PSCs with IL-6 results in sustained phosphorylation of STAT3 but does not lead to ERK activation or PSC transformation. Pancreatitis-related factors such as CTGF, TGF-β and PDGF induce the secretion of IL-11 from PSCs, and blocking IL11RA with a neutralizing antibody prevents PSC activation by these stimuli. This highlights the significant role of autocrine IL-11 activity, particularly through ERK signaling, in PSC activation. In mouse models, pancreatic IL-11 levels were elevated after pancreatic duct ligation, and in human subjects with chronic pancreatitis, both IL-11 and IL-11RA levels were elevated. Administering anti-IL11RA to mice following pancreatic duct ligation reduced pathological signaling pathways (ERK, NF-κB, STAT), pancreatic fibrosis, atrophy and levels of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6). It was the first evidence of IL-11-mediated activation of PSCs, suggesting that targeting IL-11 in the stromal cells could be a therapeutic approach for managing pancreatitis [71].
IL-11 exhibits anti-inflammatory properties in animal models of intestinal inflammation, endotoxemia, and thoracic injury caused by radiation exposure. The protective role of IL-11 in a mouse model of ANP was investigated. AP was induced by administering seven intraperitoneal injections of cerulein (50 µg/kg) at hourly intervals, followed by the injection of lipopolysaccharide (LPS) 5 h after the first cerulein injection. Recombinant human IL-11 (rhIL-11) treatment was initiated 30 min before the first cerulein injection and repeated 4 h later. However, treatment with rhIL-11 significantly reduced lipase and amylase activity at 6 and 8 h. Serum TNF-α levels peaked at 6 h and then declined sharply. The increase in circulating TNF-α was notably inhibited by rhIL-11 administration. Histologically, rhIL-11 treatment mitigated the severity of the pancreatic injury, including reductions in inflammatory cell infiltration, edema and hemorrhage at 6 h. Moreover, intrapancreatic TNF-α mRNA levels were decreased by more than 50% in the rhIL-11-treated group at 6 h. Therefore, rhIL-11 reduced the severity of experimental pancreatitis, particularly in the early stages, and suppressed intrapancreatic TNF mRNA expression in vivo. This suggests that IL-11’s favorable properties during the initial phase of AP may be, at least in part, attributed to its impact on TNF-α synthesis [72].
Both anti-inflammatory and proinflammatory cytokines have roles in the development of AP. In patients with SAP, the circulatory IL-10 levels were notably elevated from the first to the seventh day compared to those with MAP. Additionally, individuals experiencing severe attacks exhibited significantly higher serum IL-11 levels on days two to four when compared to those with mild attacks. However, no significant differences in IL-11 levels between the two groups were found on days one and seven. Such results support the validity of serum IL-11 and IL-10 as indicators of the severity of AP. Specifically, IL-10 proves to be a valuable early predictor of the AP prognosis [73].
Interleukin-12 (IL-12)
IL-12 is a cytokine made up of two subunits, p35 and p40, forming a heterodimeric structure. It is synthesized by antigen-presenting cells and plays a crucial role in defending the host against intracellular microbial infections and controlling cancer growth. IL-12 achieves this by activating both adaptive and innate immune cells. Due to its potent effects, the production of IL-12 is tightly controlled in terms of timing, location, and quantity during an immune response. Any disruption in this delicate balance can lead to immunological disorders. The discovery of new functions of IL-12 and related molecules in various immunological contexts, and the exploration of the physiological pathways involved in regulating IL-12 production improved the knowledge of IL-12’s role, and its expression pattern is likely to be beneficial in the recognition of therapeutic approaches to correct immune malfunctions [74].
Gregorić et al. [75] investigated whether serum IL-12 levels could serve as an early indicator of the severity of SAP. Additionally, the study aimed to examine the relationship between IL-12 levels, the SIRS score, the APACHE II score, and the Ranson score in predicting the severity of the illness and its outcome in SAP patients. It revealed that the concentration of IL-12 in the blood was notably higher at the 24-hour mark when compared to IL-12 levels upon admission and after 72 h. These findings suggest that serum IL-12 values could be employed as an early indicator of SAP severity and progression [75].
Pro-inflammatory cytokines are involved in the pathogenesis of AP. Researchers assessed and established a clinical correlation between the proportions of peripheral blood mononuclear cells (PBMC) containing IL-12 and IL-6. Additionally, they compared these findings with the APACHE III scores in a cohort of AP patients. The severity of AP was determined based on the Atlanta criteria. A noteworthy association was observed between IL-6 levels and APACHE scores in cases of severe AP. For IL-6-positive PBMCs, the cut-off percentage was set at >25% (resulting in a positive predictive value (PPV) of 100%), while for IL-12-positive PBMCs, the cut-off percentage was > 9% (with a PPV of 70%). These findings suggest that it is reasonable to combine the APACHE III score with the percentage of IL-6 in PBMCs to assess the severity of AP [76].
The patients with AP displayed significantly higher serum levels of IL-12, IL-12p40, and IL-6 in comparison with the healthy counterparts from the first day to the sixth day. On the first day of the disease, AP subjects exhibited significantly higher serum levels of IL-12p70 than the healthy subjects, but these levels significantly decreased on the second, third, and fourth days of illness. It is important to note that the reduction in IL-12p70 levels among AP patients was not attributed to monocyte dysfunction, as evidenced by the increased production of IL-6. The elevated levels of IL-12p40 in AP patients may contribute to their increased susceptibility to infections [77]. IL-12 and IL-18 are two proinflammatory cytokines that stimulate the Th1 cell response, marked by elevated IFN-γ levels. Monocytes/macrophages are the primary sources of these cytokines. In individuals with AP, both pancreatic and serum concentration of IL-18 and IL-12 are increased, and this increase is linked to the disease severity. The increased levels of IL-12 and IL-18 are likely the cause of increased IFN-γ levels in the bloodstream and its expression by CD8+ lymphocytes that infiltrate the pancreas in patients with SAP [78].
Interleukin-15 (IL-15)
IL-15 interacts with the IL-15-specific high-affinity binding protein IL-15Rα, and initiates signaling through a complex involving a β chain and an γ chain. This complex leads to the recruitment of JAK1 by the β chain and activates JAK3, which is inherently linked with the γ chain [79]. Mature human IL-15 is a 14–15 kDa glycoprotein and a member of the four α-helix bundle cytokine family [80, 81]. A diverse array of cell types consistently produces IL-15 mRNA, including macrophages, monocytes, dendritic cells (DCs), epidermal skin cells, keratinocytes, different types of epithelial cells, fibroblasts, nerve cells, and bone marrow stromal cells [79, 82].
In SAP, the occurrence of multiple OD syndrome (MODS) significantly contributes to a high mortality rate. Through Western blot analysis, the expression of IL-15 in the pancreas, lung, liver and intestine during pancreatitis induced by 3% sodium deoxycholate (DCA) was observed. Immunohistochemical staining further confirmed the presence of IL-15 in the cytoplasm of each of these organs. Notably, when recombinant IL-15 protein was administered during 3% DCA-induced pancreatitis, it mitigated the rise in serum alanine aminotransferase (ALT) levels and improved lung morphology 18 h after SAP induction. Additionally, in cases of 20% DCA-induced pancreatitis, IL-15 was found to ameliorate the increase of serum ALT and amylase activity just 6 h after SAP induction. These results strongly support the notion that IL-15 plays an important role in the development of organ dysfunction in SAP and acts as a protective biomarker against organ injuries [83].
Interleukin-17 (IL-17)
Among cytokines, IL-17 stands out as a crucial proinflammatory biomolecule generated by T helper 17 (Th17) cells, natural killer cells and γδ T cells. Its primary function revolves around orchestrating responses to pathogens and symbiotic interactions, influencing a range of targets aimed at striking a balance between the immune system’s inflammatory response and its overall functioning. Alongside its potential role in fine-tuning the immune-inflammatory response to maintain an equilibrium between tolerant and cytotoxic immune profiles, IL-17 can also contribute to acute tissue damage. Serving as a proinflammatory mediator, IL-17 is an integral part of a complex network of cytokines that play a crucial role in various inflammatory conditions and the development of diseases [84–89].
IL-17 initiates damage to acinar cells of the pancreas by producing and releasing chemokines and cytokines. These substances serve to attract immune cells (i.e., neutrophils and macrophages) to the affected area. Additionally, IL-17 directly prompts the production of chemokines and activates innate immune cells, which in turn release pro-inflammatory agents. Different inflammatory factors work in conjunction, exacerbating the condition of AP. Moreover, signals from the intestinal microbiota play a role in IL-17 activation. These signals encompass alterations in the microbiota composition, metabolites derived from bacteria, and impaired function of the intestinal barrier. The involvement of IL-17 in the inflammatory response favors the early onset of systemic injury, which may precede tissue necrosis. Subsequently, late-stage secondary organ failure can result from infected pancreatic necrosis (IPN), ultimately culminating in sepsis [90].
AP is characterized by different clinical phases. It begins with an initial SIRS, and in some cases, there is a consequent ‘second hit,’ typically triggered by systemic sepsis. The pro-inflammatory T-helper 17 pathway has been identified as the initiator of the early SIRS in AP. However, so far, IL-17A has not been assessed as a biomarker for the septic second hit in SAP. There was no statistically significant difference between the groups. Consequently, the IL-17A concentration did not serve as a statistically significant identifier of the second hit in AP [91].
In the same way, IL-17A is a proinflammatory cytokine that has garnered significant attention recently. Its role in different inflammatory disorders is confirmed. However, its specific function in AP has remained uncertain. The role of IL-17A in experimental ANP was assessed. A significant increase in the expression of IL-17A following experimental AP was detected. Furthermore, recombinant rat IL-17A was found to induce necrosis in rat acinar cells of the pancreas and stimulate the expression of several target genes, among them CXCL1, CXCL2, CXCL5, IL-1β and IL-6, in both stellate and acinar cells of the pancreas. These results suggest that IL-17A may play a role in causing damage of the pancreas via regulation of the expression of proinflammatory chemokines and cytokines and during experimental AP [92].
SAP is characterized by systemic inflammation, immune suppression, and the potential development of sepsis, which can ultimately result in vital organ failure and mortality. It was found that an earlier and more pronounced increase in serum IL-17 levels was indicative of prolonged hospitalization, organ failure and a higher risk of mortality, potentially due to its impact on disrupting the integrity of the gut barrier. Continuous veno-venous hemofiltration was found to be capable of removing inflammatory cytokines from the serum, including IL-6 and IL-17. This removal process helped to dampen the inflammatory response and reduce related systemic complications [93].
In combination with TNF-α, IL-17A plays a crucial role in promoting inflammatory responses. Disrupting the cooperative action of these two cytokines is a reliable strategy for managing inflammatory disorders. Ellipticine, a naturally occurring alkaloid, possesses anti-tumor and anti-HIV properties. However, it is currently unclear whether ellipticine can inhibit IL-17A and TNF-α-mediated signaling and whether it has therapeutic effects on post-acute lung injury (PALI). It was observed that ellipticine notably suppressed the secretion of pro-inflammatory chemokines and cytokines in pulmonary epithelial cells (BEAS-2B) when exposed to IL-17A and TNF-α, but not when treated with either TNF-α or IL-17A alone. Additionally, ellipticine mitigated the activation of MAPKs and NF-κB in response to TNF-α and IL-17A, led to inhibition of Act1 and TRAF6-mediated NF-κB activation, and disrupted the interaction between Act1 and TRAF6. Furthermore, it was found that ellipticine significantly reduced both caerulein and LPS-induced SAP and PALI. Ellipticine treatment resulted in a substantial reduction in inflammatory cell infiltration, serum myeloperoxidase (MPO), lipase and amylase activity, as well as the protein concentration in bronchoalveolar lavage fluid (BALF). These findings suggest that ellipticine hampers the synergistic effects of TNF-α and IL-17A by targeting the interaction between TRAF6 and Act1, making it a potential therapeutic agent for the treatment of SAP/PALI [94].
Interleukin-18 (IL-18)
Initially recognized as a factor that induces IFN-γ production, IL-18 has been found to play a role in both Th1 and Th2 immune responses, as well as in the activation of macrophages and natural killer cells. IL-18 is believed to play an important role in various inflammatory conditions, such as Crohn’s disease, cancer, chronic obstructive pulmonary disease, etc. Recently, it has become clear that IL-18 also has a specific role in pancreatic diseases, such as cancer, AP, and chronic pancreatitis [95].
Levels of IL-18 serve as markers for assessing AP severity and exhibit a notable inverse correlation with the levels of protective factors against oxidative damage, such as serum glutathione peroxidase (GPx) and selenium. The interplay between IL-18 and other pro-inflammatory biomolecules underscores that IL-18 is a major mediator of inflammation in the pathogenesis of AP. Elevated serum IL-18 levels may contribute to liver injury associated with AP. Emerging strategies for therapeutic intervention in AP include the administration of IL-18 antagonists to directly inhibit the activity of IL-18. Additionally, indirect approaches involve P2X7 receptor antagonists and ICE inhibitors to thwart IL-18 activity. These approaches assume that targeting IL-18 specifically holds promise as a therapeutic avenue for managing AP [96].
IL-18 is primarily secreted by macrophages, with a notable contribution from liver Kupffer cells. When anti-IL-18 antibodies were applied before exposing subjects to LPS 1 week after Propionibacterium acnes injection, it effectively prevented the onset of hepatic necrosis and the subsequent elevation in serum AST and ALT activity. Consequently, its investigation shed light on the connection between serum IL-18 levels, disease severity, and the onset of hepatic disorder in AP cases. A significant rise in IL-18 concentrations corresponded with the AP severity. Furthermore, the group experiencing complications related to MODS exhibited significantly higher IL-18 values compared to those without such complications. It is worth noting that the correlation between the severity of AP and serum IL-18 levels may not be exclusive to AP, as similar correlations with APACHE II scores were detected in patients with sepsis [97].
Oxidative stress is a significant contributor to an inadequate immune response during the initial stages of AP. In both mild and severe forms of AP, the concentration of IL-18 in the serum was notably higher compared to that in the healthy control group. Additionally, a significant reduction in GPx concentration in the serum of SAP patients when compared to those with the mild form of the condition and the control group was observed. Furthermore, the selenium concentration in the SAP cases was significantly lower. Significant correlations between IL-18, GPx and selenium were observed. The ROC demonstrated that IL-18 and GPx have a high prognostic accuracy in determining the severity of AP. IL-18 is secreted in the early phases of AP and may play a pivotal role as an immunomodulator in the inflammatory response during the severe form of AP. The reduced levels of GPx and selenium in SAP reflect diminished antioxidative capacity in AP. Consequently, IL-18 and GPx could potentially serve as novel indicators for determination of the severity of AP [98].
The release of the immune-modulating factor IL-18 into the bloodstream at the first step of AP correlates with the severity of the disease. IL-18 triggers the production of nitric oxide (NO), which is implicated in the pathophysiology of AP. Another study aimed to elucidate the role of IL-18 in the development of the disease and NO production during the early stages of AP, using recombinant mouse (rm) IL-18 protein and IL-18 gene KO mice. In the experiment, mice were pretreated with either phosphate-buffered saline or rmIL-18 and then injected intraperitoneally with either phosphate-buffered saline (sham) or cerulein (AP) hourly for 3 h. The primary measurements included serum IL-18 levels, lipase and amylase activity, histological assessment of the pancreas with a focus on vacuolization of acinar cells, mRNA expression of inducible nitric oxide synthase (iNOS) in the pancreas, spleen, and liver, and the levels of NO metabolites in the plasma. In wild-type (WT) mice, serum IL-18 significantly increased immediately after the induction of AP. Notably, serum amylase, lipase, and the proportion of acinar cells displaying vacuolization were higher in the AP/KO group compared to the AP/WT group. However, these biomarkers were ameliorated with dose-dependent pretreatment using rmIL-18 in both groups. Expression of the pancreatic iNOS gene and plasma NO metabolite levels significantly increased within 6 h after AP initiation but were significantly lower in the AP/KO group compared to AP/WT mice. Pretreatment with rmIL-18 also significantly increased these biomarkers in both groups. Furthermore, the expression of iNOS in the spleen and liver remained unchanged following AP induction in WT mice, but pretreatment with rmIL-18 elevated examined parameters. When an iNOS inhibitor called aminoguanidine was administered before AP induction, it nullified the protective effect of rmIL-18 pretreatment on pancreatic injury. In summary, IL-18 seems to safeguard the pancreas during the early stages of experimentally induced AP in mice, likely by inducing the release of NO from an iNOS source. These results suggest that IL-18 may hold promise as a target for potential therapeutics in the treatment of AP [99].
AP continues to require improved diagnostic and therapeutic approaches to reduce its associated mortality and morbidity. It seems that assessing serum levels of IL-18 and examining their correlation with CRP could offer valuable prognostic insights. A positive correlation was observed between serum IL-18 and CRP levels, with this association slightly increasing over the course of several days. It was observed that the serum IL-18 level rises during the initial stage of AP, suggesting its potential use as a marker for assessing the inflammatory response in subjects with AP. Moreover, its correlation with CRP indicates a potential prognostic role for IL-18 in AP [100].
The results indicated that the concentration of IL-18 increased in SAP patients during the first 24 h after the onset of pain and was notably higher than in MAP subjects and healthy counterparts. The AUC analysis revealed that the prognostic accuracy of IL-18 (AUC = 0.722) was comparable to that of the APACHE II score (0.729), both of which were superior to CRP (0.531) in differentiating between mild AP and SAP. In conclusion, IL-18 can function as an early predictor and aid in the diagnosis of SAP within the first 24 h following the onset of pain or admission. It is a reliable, convenient, and swift diagnostic tool. However, it should be emphasized that IL-18 alone is insufficient for a definitive AP diagnosis, and additional tests are necessary for confirmation [101].
A study was conducted to examine how administering two injections of IL-12 along with IL-18 to mice affects pancreatic inflammation, taking into consideration the role of leptin and obesity. It indicated that when obesity is coupled with a lack of leptin, it amplifies the pancreas’s susceptibility to the harmful effects of the IL-12 and IL-18 combination. Interestingly, it appears that the escalation in the AP severity in leptin-deficient ob/ob mice is primarily due to obesity itself rather than the absence of leptin. The researchers developed a disease model that closely resembles the pathological aspects of AP and obesity-related adipose tissue damage. This model might be a valuable tool for exploring the connection between increased body fat and the aggravation of AP in humans [102].
In another study, the serum levels of antimitochondrial antibody (AMA) showed a significant increase at the 6-hour mark but decreased at the 48-hour mark after the onset of AP. Notably, the rise in IFN-γ was more pronounced than that observed for AMA. In the AP and IFN treatment groups, IL-18 levels increased, with a particularly marked increase in IFN-γ at the 48-hour time point after AP onset. In contrast, IL-27 levels decreased 24 h after AP in the IFN group compared to the AP group. In the AP group, there was an increase in the immunostaining of cytokines. However, in the IFN treatment group, more severe pancreatic edema was observed, and both IL-18 and NF-κB expression were higher as compared to the other groups. It suggested that IFN-γ has the ability to raise circulating IL-18 levels and decrease IL-27 levels in AP. This elevation in IL-18 and NF-κB may influence other pro-inflammatory mediators, exacerbating AP. Therefore, controlling IFN-γ may hold promise as a strategy to mitigate pancreatitis [103].
Regardless of the underlying cause, the inflammatory response in severe cases of AP is quite similar. Although the specific triggers of AP are not completely understood, cytokines are recognized as crucial mediators in the pathophysiology of SAP. Surprisingly, only a limited number of studies have explored the roles of IL-18 and intercellular adhesion molecule 1 (ICAM-1) in human AP. Necrotizing pancreatitis was linked with increased levels of IL-18 and soluble ICAM-1 (sICAM-1). In subjects with a non-complicated course of the disease, IL-18 concentrations decreased after the first week, whereas they remained elevated in patients with persistent MODS and infection of necrotic tissue. Peak concentrations of sICAM-1 were recorded at the time of admission, followed by a gradual decrease during the period of observation. These biomarkers exhibited correlations with the disease severity, the onset/progression of MODS, and the presence of infected necrotic tissue. The mentioned proinflammatory biomarkers appear to play a role in the development of SAP, and they are closely linked with the disease severity. Consequently, they may have potential as prognostic markers for assessing the course and outcome of the condition [104].
Interleukin-19 (IL-19)
IL-19 was initially discovered through a similarity in its genetic sequence to IL-10 and belongs to the IL-10 family. This family also comprises IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B, and IL-29. Previous research has demonstrated that different types of cells, such as keratinocytes, epithelial cells, macrophages, B cells, vascular smooth muscle cells, etc., can produce IL-19. Pancreatitis arises due to inflammation, tissue damage and fibrosis, particularly affecting acinar cells of pancreas. Fujimoto et al. [105] utilized an AP experimental animal model to investigate the role of IL-19 in the inflammatory state in the pancreas. Through targeting of genes, it produced mice lacking IL-19 (IL-19 KO mice). To assess tissue destruction, it induced an AP animal model by cerulein, an analogue of cholecystokinin. When administered to the mice, cerulein excessively stimulated the secretion of digestive enzymes, leading to AP. It revealed that cerulein-induced AP was more severe in IL-19 KO mice. This increased AP severity linked with heightened levels of apoptosis. Thus, it firmly indicated that IL-19 plays a crucial role in regulating apoptosis during cerulein-induced AP [105] (Table I).
Conclusions
Interleukins are important in AP since they are key regulators of the inflammatory response, tissue damage, and disease severity. Understanding their role in the context of pancreatitis can lead to better diagnostic and therapeutic strategies for managing this condition. This article confirms the significant role of IL-1, 2, 4, 5, 6, 8, 10, 11, 12, 15, 17, 18, 19 in the pathogenesis of AP. Additional research is necessary to elucidate the role of IL-3, 7, 9, 13, 14, 16, 20 in AP. Researchers are exploring ways to modulate interleukin activity to reduce inflammation and improve outcomes in individuals with this condition. Drugs that target specific interleukins might be developed to mitigate the effects of AP.