Introduction
Primary hepatocellular carcinoma (HCC) is a highly malignant tumour with a high incidence in Asian countries. The pathogenesis of HCC is complicated, and multiple signal transductions are involved in the carcinogenesis and tumour progression [1]. However, HCC is not easily detected in the early stage. A considerable number of patients are already burdened with moderate to advanced stages after diagnosis, and the treatment is extremely difficult. Only 10% to 30% of HCC patients can undergo surgery in the clinic. Transcatheter arterial chemoembolization (TACE), radiofrequency ablation, and other treatments are generally performed in patients who cannot undergo surgery, while chemotherapy and targeted therapy are conducted in the majority of patients [2, 3]. At present, the first choice of chemotherapeutic drugs in HCC treatment is cytotoxic drugs such as gemcitabine and fluorouracil, which are accompanied with heavy side effects and lack of reproducibility [4]. Therefore, targeted therapy is gradually being promoted at present.
Sunitinib is a novel oral small molecule, multi-targeted tyrosine kinase inhibitor. It has been proven that it harbours anti-tumour angiogenesis and anti-tumour activity. Its mechanism is mainly via inhibition of members of the receptor tyrosine kinase (RTK) family, including vascular endothelial growth factor receptor (VEGFR-1/2), platelet-derived growth factor receptor (FDGFR-α/β), foetal liver tyrosine kinase receptor (FLT3), and colony-stimulating factor 1 (CSF-1), etc. [5]. Clinical trials have verified that sunitinib has significant clinical efficacy on gastrointestinal stromal tumours (GIST), metastatic renal cell carcinoma, and HCC, which can prolong overall survival (OS) and progression-free survival (PFS) [6]. However, similar with other targeted drugs, there are obvious individual differences of sunitinib efficacy, with unrevealed reasons for this individualized difference.
Long non-coding RNA (lncRNA) is a member of the non-coding RNA (nc RNA) family. More and more studies have demonstrated that lncRNA is closely associated with tumour progression and chemotherapeutic resistance, etc. [7]. The lncRNA HOTAIR study has shown that it is highly expressed in multiple malignant tumours, which can be used as a prognostic marker of breast cancer and be utilized to predict the prognosis of HCC transplantation. Additionally, other studies have shown that lncRNA HOTAIR is correlated with drug resistance of cisplatin, trastuzumab, etc. Therefore, in this study, from a clinical perspective, we assessed the expression of lncRNA HOTAIR in advanced HCC patients, and we investigated the correlation of lncRNA HOTAIR expression with prognosis following sunitinib therapy, aiming to provide reference and support for personalized medicine in clinical practice.
Material and methods
Origins of cases
Of the 60 patients enrolled in this study, all were diagnosed with advanced HCC (TNM stage III and IV). All patients did not receive any anti-tumour therapy when they were enrolled, including chemotherapy, radiotherapy, or biological therapy. Patients were confirmed by medical history, signs, laboratory examinations, and imaging examinations. These patients were unable to undergo surgical resection or local treatment. The histological tissue obtained by needle biopsy was confirmed as stage III and IV according to the International Union Against Cancer and the American Joint Committee on Cancer TNM staging classification (seventh edition). Among the 60 patients, there were 35 males and 25 females. Before the treatment, the ECOG score of the patients was 0-2, with B or C of Barcelona staging and A or B of Child-Pugh liver function.
Sixty patients were treated with sunitinib monotherapy. All patients were treated with sunitinib malate (Pfizer). In brief, sunitinib was orally administered at a dose of 37.5 mg once daily for 4 consecutive weeks. In the case of drug-related dose-limiting side effects, treatment can be reduced or discontinued, according to the patient’s tolerance, until the emergence of disease progression or death. The dose reduction was performed at a gradient dose of 12.5 mg based on the type and severity of adverse reactions. In the case of 2 consecutive adverse reactions of Grade 3, the drug dose could be reduced by 1 level. In the case of Grade 4 adverse reactions, sunitinib administration was ceased, while the patient was re-administered with sunitinib by decreasing by 1 dose level after recovery. The minimum dose was 12.5 mg in all patients, and in the case of another intolerance, they would be permanently discontinued. All patients signed written informed consent before receiving treatment. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Jiaxing University.
The extraction of total RNA from peripheral blood and tumour tissue of patients
Peripheral blood and tumour tissue was obtained from 50 patients before treatment. The extraction of total RNA from peripheral blood was as follows. After the patient was fasted for 12 h, 5 ml of peripheral blood was collected at the elbow, which was placed in a heparin anticoagulant tube. After dilution with the same dose of PBS, mixing by pipetting at room temperature, a 1/2 diluted volume of lymphocyte separation solution (Histopaque-1077, Sigma, USA) was added to the centrifuge tube, added slowly along the wall of the tube, followed by centrifugation at 3000 rpm for 30 min to obtain peripheral blood mononuclear cells (PBMCs). The EZNA Blood RNA Mini Kit (Omega, US) was used to extract RNA from PBMCs. Total RNA was extracted and stored at –80°C, and cDNA was synthesized within 1 week and stored at –20°C. The extraction of RNA from tumour tissue was as follows. Briefly, a small amount of liquid nitrogen was added to the mortar, and we waited until the liquid nitrogen volatilized to completely pre-cool the mortar. An appropriate amount of liquid nitrogen was added, and tumour tissue was subsequently added to grind it, followed by the addition of 1 ml of TRIzol after powdering. After the tissue was completely dissolved, the homogenate was transferred to an EP tube. Afterwards, an Invitrogen TRIzol kit (Invitrogen, US) was used to extract RNA, which was stored at –80°C, and cDNA was synthesized within 1 week and stored at –20°C.
Real-time quantitative PCR (RT-qPCR)
Total RNA was reverse-transcribed using the PrimeScriptTM RT reagent kit with gDNA Eraser kit (Takara, China). In the reaction system, 1 μg of RNA was contained in per 20 μl of the system. The reverse transcription was performed as follows: 7 μl of 60 ng/μl RNA, 2 μl of 5×gDNA Eraser buffer, and 1 μl of gDNA Eraser were mixed and reacted for 1 min at 45°C, followed by the addition of 4 μl of 5×PrimeScript Enzyme Buffer, 1 μl of PrimeScript Enzyme, 1 μl of PrimeScript RT Mix, and 4 μl of nuclease-free H2O, which were mixed and incubated at 37°C for 15 min to obtain cDNAs. The synthesized cDNA was amplified using SYBR Premix Ex Taq II reagent (Takara, China) in a 10-μl reaction system. The reagents and procedures of qPCR were as follows: 5 μl of 2×SYBR®Premix Ex TaqTM and 0.2 μl Primer Forward (10 pmol/μl), 0.2 μl Primer Reverse (10 pmol/μl), 0.2 μl ROX Dye II, and 2.4 μl Nuclease-free H2O were mixed and amplified using ABI step one. The reaction conditions were as follows: 95°C for 2 min, 40 cycles of 15 s at 95°C and 30 s at 58°C; the fluorescence was collected at 58°C. The dissolution temperature was gradually increased from 58°C to 95°C, and fluorescence was collected by every 0.5°C increase. The lncRNA HOTAIR forward primer: 5’-GCCTGAACTTCCTCCTGCTATT-3’, the reverse primer 5’-ACAC-AAAGTGCATACCTACCCA-3’ (329 bp in length). The internal reference β-actin forward primer 5’-AAGAGAGGCATCCTCACCCT-3’, the reverse primer 5’-TACATGGCTGGGGTGTTGAA-3’ (216 bp in length).
Statistical analysis
SPSS19.0 software was used for statistical analysis. The measurement data were compared by t-test, analysis of variance and Mann-Whitney U test. The survival analysis was conducted by Kaplan-Meier method, followed by log-rank test for the comparison between groups. Cox regression analysis was used for univariate and multivariate analyses. P < 0.05 was considered as statistical significance.
Results
Basic information of the patients
A total of 60 patients were enrolled in this study, who were admitted and diagnosed with advanced-stage HCC at the Second Affiliated Hospital of Jiaxing University from January 2015 to December 2016. Among them, 35 were male and 25 were female, 38 were in stage III, and 22 were in stage IV. The median OS was 10.4 months (range: 6.5–18.7 months), and the median PFS was 6.8 (range: 3.8–11.6). The basic information of patients is shown in Table I.
The expression level of lncRNA HOTAIR in tumour tissue and peripheral blood of patients
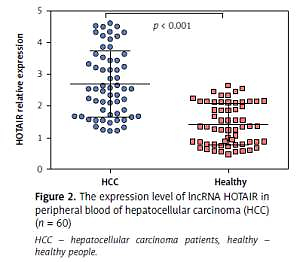
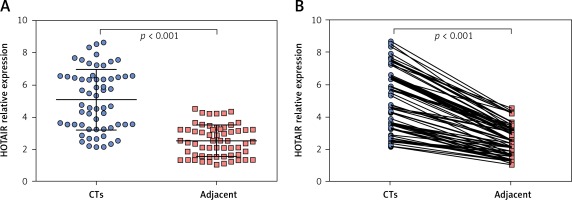
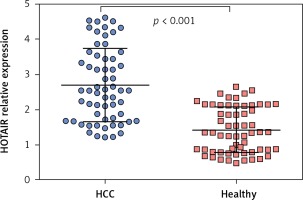
The average expression level of lncRNA HOTAIR in tumour tissues of HCC patients was 5.07 (range: 2.14–8.65, mean: 5.07 ±1.74), and the average expression level of lncRNA HOTAIR in adjacent tissues was 2.53 (range: 4.52–1.04, mean: 2.53 ±0.89). The level of lncRNA HOTAIR in tumour tissues was significantly higher than that in adjacent tissues (t = 9.03, p < 0.001), as shown in Figures 1 A and B. The average expression level of lncRNA HOTAIR in peripheral blood of HCC patients was 2.69 (range: 4.62–1.21, mean: 2.69 ±0.89), and the average expression level of lncRNA HOTAIR in peripheral blood of healthy individuals was 1.41 (range: 2.64–0.67, mean: 1.41 ±0.48), which were statistically different (t = 8.04, p < 0.001). The results are shown in Figure 2. The expression level of lncRNA HOTAIR in tumour tissue and peripheral blood of HCC patients was correlated (r = 0.638, p < 0.001).
The correlation of the expression level of lncRNA HOTAIR with clinicopathological characteristics
The median of lncRNA HOTAIR expression in tumour tissues was 5.1, and the median of lncRNA HOTAIR expression in peripheral blood was 2.7. The medians were used as cut-offs, and patients were divided into a high-expression group (High) and a low-expression group (Low). The Mann-Whitney U test revealed that the expression level of lncRNA HOTAIR was not correlated with clinicopathological characteristics, including age (p = 0.73, 0.93), gender (p = 0.38, 0.71), drinking history (p = 0.31, 0.31), physical status (p = 0.81, 0.78), tumour stage (p = 0.10, 0.23), and cirrhosis history (p = 0.69, 0.46) (shown in Table II).
Table II
The correlation between lncRNA HOTAIR expression and the clinicopathological characteristics of patients (n %)
The correlation of OS and PFS with the expression level of lncRNA HOTAIR in tumour tissue and peripheral blood
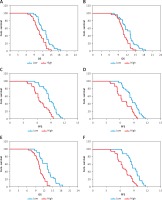
The median OS of patients with low and high lncRNA HOTAIR expression in tumour tissue was 13.4 vs. 9.5 (log-rank 17.9, p < 0.001, Figure 3 A). The median OS of patients with low and high expression of lncRNA HOTAIR in peripheral blood was 12.8 vs. 9.1 (log-rank 16.3, p < 0.001, Figure 3 B). The median PFS of patients with low and high lncRNA HOTAIR expression in tumour tissues was 8.4 vs. 6.2 (log-rank 19.7, p < 0.001, Figure 3 C). The median PFS in patients with low and high lncRNA HOTAIR expression in peripheral blood was 8.9 vs. 6.4 (log-rank 18.3, p < 0.001, Figure 3 D). The median OS of patients with low expression of lncRNA in both tumours and peripheral blood and the rest of the patients was 14.3 vs. 8.8 (log-rank 21.8, p < 0.001, Figure 3 E). The median PFS of patients with low expression of lncRNA in both tumours and peripheral blood and the rest patients was 10.6 vs. 6.0 (log-rank 20.4, p < 0.001, Figure 3 F). The results were shown in Figure 3.
Correlation between OS and clinicopathological characteristics
The clinical stage and physical status of HCC patients were correlated with their OS (log-rank: 6.571, 5.351, p = 0.013, 0.018). COX regression analysis demonstrated that patients with low expression of lncRNA HOTAIR in tumour tissues or peripheral blood had longer OS (HR = 4.035, 4.135, p = 0.04, and 0.031). Patients with low expression of lncRNA HOTAIR in both tumour tissue and peripheral blood had further prolonged OS (HR = 9.873, p = 0.001). The results are shown in Table III.
Table III
The correlation between OS and clinicopathological characteristics
Correlation between PFS and clinicopathological characteristics
The clinical stage and physical status of patients were correlated with their PFS (log-rank: 7.685, 6.354, p = 0.031, 0.038). COX regression analysis revealed that patients with low expression of lncRNA HOTAIR in tissues or peripheral blood had longer PFS (HR = 3.852, 3.168, p = 0.025, 0.026). Patients with low expression of lncRNA HOTAIR in both tumour tissue and peripheral blood had prolonged PFS (HR = 9.341, p = 0.001). The results are shown in Table IV.
Table IV
The correlation between PFS and clinicopathological characteristics
Incidence of adverse reactions during sunitinib treatment
Digestive tract adverse reactions include the following: nausea, vomiting, diarrhoea, indigestion, constipation, etc. A total of 12 patients developed such adverse reactions, all of which were grade I, with an incidence of 20%. Two patients had abnormal liver function and recovered after drug reduction. One patient developed a rash and resumed after withdrawal, and no obvious adverse reactions were found in other patients. The incidence of gastrointestinal adverse reactions of sunitinib is the highest, but the degree is low, and patients can tolerate it.
Discussion
Primary liver cancer is a common malignancy and is particularly prevalent in Southeast Asian countries. HCC is one type of primary liver cancer with relatively high prevalence. In particular, a considerable proportion of patients are already in advanced stages when they are diagnosed. For those patients, treatment is relatively tough, and the prognosis is relatively poor. At present, great attention has been paid to multi-kinase inhibitor targeted drugs as therapeutic approaches to HCC. Currently, targeted drugs for HCC include somatonin, sunitinib, etc. [8].
Sunitinib is a multi-kinase small molecule compound that inhibits tyrosine kinase targets such as VEGFR (VEGFR1-3) and PDGFR. In a Phase II, multicentre, European/Asian clinical trial in 2006, the median OS was 44 weeks and the TPP was 21 weeks, in patients undergoing sunitinib monotherapy. According to Response Evaluation Criteria in Solid Tumours (RECIST), over 30% of tumours underwent necrosis after 1–1.5 months of treatment, indicating that sunitinib exerted an anti-hepatocarcinogenic effect [9]. The second phase II clinical trial showed [10] that of the 45 patients with advanced HCC treated with 37.5 mg/day sunitinib, 15 patients were still alive and had no significant progress after 12 weeks. The median TPP was 3.2 months, and the median OS was 9.3 months, which was consistent with our findings (median OS: 10.4 months, ranging from 6.5 to 18.7 months, median PFS: 6.8, ranging from 3.8 to 11.6 months), suggesting that sunitinib has little difference in overall efficacy in HCC therapy, and consistency with previous studies. However, in previous studies, we found that the therapeutic efficacy of sunitinib in HCC patients was quite different. In addition, there is no study concerning the efficacy prediction and evaluation. We speculate that it might be related to HCC progression, primary resistance of patients, acquired drug resistance, and adaptive drug resistance.
In the study of tumours, there have been extensive studies concerning non-coding RNA as a marker of tumour prognosis, especially lncRNA. lncRNA HOTAIR is relatively mature in the study of tumours. The present reports demonstrate that the expression of lncRNA HOTAIR is high in a variety of tumours. Gupta et al. have reported that lncRNA HOTAIR is an important prognostic indicator in primary breast cancer, and lncRNA HOTAIR can regulate tumour metastasis [11]. Kogo et al. [12] found that lncRNA HOTAIR plays a critical role in stage IV colorectal cancer, confirming that the expression of lncRNA HOTAIR in colorectal cancer is higher than that in adjacent tissues. Yang et al. [13] found that the increased expression of lncRNA HOTAIR is associated with tumour recurrence following liver transplantation in HCC patients, which can be used as an independent prognostic factor. In the study of drug resistance, the sensitivity of tumour cells to cisplatin and doxorubicin is correlated with the expression level of lncRNA HOTAIR [14, 15], indicating that lncRNA HOTAIR not only serve as a biomarker for tumour prognosis but also as a potential therapeutic target.
From the results of this study, the expression of lncRNA HOTAIR is high in patients with advanced HCC, and this high expression of lncRNA HOTAIR is not correlated with clinicopathological characteristics, and the expression level of lncRNA HOTAIR in tumour tissue and peripheral blood is correlated (r = 0.638, p < 0.001), which are consistent with previous studies, indicating that lncRNA HOTAIR also plays an important role in HCC. In the analysis of survival, patients with low expression of lncRNA HOTAIR in tumour tissue and peripheral blood are more likely to benefit from sunitinib therapy, obtaining longer OS and PFS. In addition, COX regression analysis showed that lncRNA HOTAIR was an independent prognostic factor for sunitinib in treating advanced HCC.
Collectively, we demonstrate that lncRNA HOTAIR is highly expressed in tissues and peripheral blood of patients with advanced HCC, and lncRNA HOTAIR can be used as an independent prognostic factor for sunitinib in treating advanced HCC. However, the exact conclusions require a larger sample size to be verified, and how lncRNA HOTAIR affects the efficacy of sunitinib remains to be further studied. In summary, this study provides a new idea for the individualized therapy of sunitinib in clinical practice.
In conclusion, lncRNA HOTAIR is highly expressed in tissues and peripheral blood of patients with advanced HCC, and lncRNA HOTAIR can be used as an independent prognostic factor for sunitinib in treating advanced HCC.