Introduction
The prevalence of heart failure (HF) is estimated to be up to 2% of the adult population [1]. In 2017, the global number of HF cases was 64.3 million [2]. In Europe alone, more than 10 million people are affected, making the issue of HF an important public health problem. The course of HF is influenced not only by the underlying aetiology and structural changes of the heart, but also by the very long list of co-morbidities, which are extremely common in HF. Important co-morbidities include hypertension and coronary artery disease, which together account for HF development in more than 50% of cases, as well as chronic obstructive pulmonary disease (COPD), chronic kidney disease, iron deficiency, anaemia, diabetes mellitus amongst many others [3].
Body wasting has been identified as having important implications for functional status, quality of life, morbidity, and mortality. The term body wasting is an umbrella term for different forms of tissue loss. Cachexia as the oldest description of body wasting, the term having been coined in ancient Greece [4], is defined as a ‘complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass’. Its major clinical feature is loss of oedema-free body weight exceeding 5% of body weight during the previous 12 months or less [1]. In the context of HF, cachexia can be termed cardiac cachexia. It may occur in 5–15% of patients with HF, especially those with reduced ejection fraction and more advanced disease status. Cardiac cachexia is associated with reduced functional capacity [5] and decreased survival [1, 6].
The term sarcopenia is defined by the presence of low muscle mass together with low muscle function, strength, or performance. The 2021 HF guidelines of the European Society of Cardiology (ESC) suggest identifying sarcopenia by the measurement of appendicular skeletal muscle mass, which was defined as the sum of the muscle mass of the four limbs. On this scale, sarcopenia is identified by a value 2 standard deviation below the mean of a healthy reference group aged 18–40 years with a cut-off value of 7.26 kg/m2 for men [1]. The article on muscle wasting cited in the HF guidelines suggests cut-off points used to detect the presence of sarcopenia as the appendicular skeletal mass index values of 7.0 kg/m2 for men and 5.5 kg/m2 for women [7]. It has been shown that patients with sarcopenic HF have reduced exercise capacity [8, 9], reduced quality of life, endothelial dysfunction [10], and reduced appetite [11] among other detrimental consequences [12]. Sarcopenia also has independent prognostic value in patients with HF [13]. It is worth noting that in 2018, the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) published a revised European consensus on the definition and diagnosis of sarcopenia, in which experts established a diagnostic algorithm and determined sarcopenia cut-off points. The group strongly emphasized that low muscle strength is the primary indicator of sarcopenia [14].
Although sarcopenia occurs as part of the ageing process (primary sarcopenia), it is accelerated by chronic diseases such as cancer or HF; this is sometimes referred to as ‘secondary sarcopenia’. In HF with reduced ejection fraction, sarcopenia can be found in 20–50% of patients and is associated with frailty [7] and increased morbidity and mortality. Sarcopenia is a major determinant of outcomes outweighing the effect of body mass index (BMI) and body weight [1]. Sarcopenia is caused and exacerbated by multiple factors such as reduced regenerative capacity, imbalance in protein turnover, alteration of fat and fibrotic composition in muscle, increased presence of reactive oxygen species, dysfunction of mitochondria and increased inflammation. Based on these mechanisms, pharmacological and non-pharmacological strategies have been developed to prevent and treat sarcopenia. Although several studies are currently in progress, no definitive treatment other than exercise training is currently available [15]. However, exercise training is an important tool to improve exercise capacity and quality of life in patients with HF, independent of the presence of sarcopenia, and should be recommended for all HF patients [16].
In the absence of recommended treatment modalities for sarcopenia in HF, we aimed to investigate the possible roles of nutraceuticals in this setting. It is important to understand that there is no internationally agreed definition of “nutraceuticals”. One possible definition was proposed by the European Nutraceutical Association, defining nutraceuticals as “nutritional products that provide health and medical benefits, including the prevention and treatment of disease” [17]. Indeed, there is evidence to suggest that some dietary supplements can contribute to the improvement of HF-related symptoms [18]. We describe and discuss the available knowledge with respect to sarcopenic HF. Due to very limited knowledge and data on the role of natural products in patients with HF muscle wasting, the authors of this paper focused mainly on data on efficacy, being highly aware of the importance of safety of nutraceuticals that should be always monitored (nutrivigilance) especially in such a difficult group of patients.
Objectives and organization of the position paper
A systematic search strategy was developed to identify randomized clinical trials (RCTs) and their meta-analyses in PubMed/Web of Science, published between January 1970 through May 2022. A literature search was performed by two authors (A.B-D. and S.v.H.) independently using MEDLINE and PubMed with the MeSH terms: “nutraceuticals”, “dietary supplements”, “herbal drug”, “heart failure”, “muscle wasting” and “sarcopenia”. The experts of the Writing Committee carefully discussed the available data on the safety and efficacy of the investigated nutraceuticals in the presented indication and then anonymously voted on the selection of those with the largest data available to be finally included in the recommendations. For each selected nutraceutical, a short description of the (possible) mechanism of action is reported, followed by the clinically observed effects and the most relevant tolerability notes.
The experts discussed and agreed on the recommended levels. The strength of recommendation of the nutraceuticals’ effect on heart failure muscle wasting has been evaluated according to a new scale prepared and recommended by the ILEP Panel, as outlined in Table I. The experts of the writing and reviewing panels completed Declaration of Interest forms where any actual and/or potential conflicts of interest were presented.
Table I
The strength of recommendation based on the quality of evidence available for each nutraceutical
While working on this Position Paper we strictly followed the International Lipid Expert Panel (ILEP) scientific policy on the preparation of the recommendations. Briefly: (1) this statement was suggested by Prof. Agata Bielecka-Dabrowa (A.B-D.), Prof. Stephan von Haehling (S.v.H.), and Prof. Maciej Banach (M.B.), and was formally sent to the Steering Committee of the ILEP (see: www.ilep.eu for details) for approval. Next, (2) official e-mails to all ILEP members were sent, inviting them to be a part of the Writing Committee (WC) of this statement, in which we also presented the tasks to be carried out and the detailed schedule regarding the work required. After establishment of the WC, (3) A.B-D. and S.v.H. started to work on the main content and scientific assumption of the paper, which were next presented to the members of the WC (using online platforms and via e-mails). Next, (4) together with selected members of the WC, we worked on the draft version of the recommendations, which were next extensively discussed with all the WC members, putting special emphasis on the management figures and tables with recommendations. In case of disagreement, each recommendation was voted on. In the next step (5), the final draft of recommendations was sent to all ILEP members for the internal review process and approval. Each comment and suggestion from the ILEP members were considered and discussed.
Nutraceuticals with anti-inflammatory properties and their potential usefulness in sarcopenic heart failure
n-3 PUFA
Omega-3 fatty acids (ω-3) are polyunsaturated fatty acids (PUFA), which are a family of essential fatty acids that mediate numerous biological processes. There are three major dietary forms of n-3 PUFA: eicosapentaenoic acid (EPA; 20:5n-3), docosahexaenoic acid (DHA; 22:6n-3) and α-linolenic acid (ALA; 18:3n-3). ALA is considered an essential dietary fatty acid, which means that it cannot be synthesized by humans [19]. A limited fraction of ALA can be converted to EPA and DHA [20]. For humans, the primary source of PUFA is fish oil. Omega-3 fatty acids are well-known for their anti-inflammatory properties and their role in the development and maintenance of neurocerebral functions [20]. As pro-inflammatory cytokines have been linked to skeletal muscle wasting, the anti-inflammatory effects of n-3 PUFA may be beneficial in the prevention of muscle mass and strength loss associated with aging, frailty, and sarcopenia. Furthermore, omega-3 fatty acids may themselves modulate muscle protein synthesis, promoting muscle strength and function. This may occur as a result of their incorporation into intracellular organelles and membrane phospholipids of the sarcolemma [21].
There is evidence that consumption of PUFA may improve the prognosis of patients with HF, particularly after myocardial infarction [18]. Multiple mechanisms of action have been considered, including: 1) anti-inflammatory properties of omega-3 fatty acids, which reduce cardiac remodelling caused by excessive interstitial fibrosis and systemic inflammatory process leading to cardiac cachexia; 2) metabolic alterations in cardiomyocytes accompanied by mitochondrial functional modification; 3) direct and/or indirect functional modification of cardiac ion channels to reduce susceptibility for fatal arrhythmia; 4) anti-hypertensive effects originating from improved vascular endothelial response; and 5) modulation of autonomic nervous system activity [22].
In the International Lipid Expert Panel position paper on impact of nutraceuticals on markers of systemic inflammation the experts made a Class I Level A recommendation for omega-3 fatty acids [23]. The authors stressed that the RCT of an EPA + DHA therapy (2.5 g/day) resulted with reduced interleukin (IL) 6 (IL-6), IL-1β and tumor necrosis factor α (TNF-α) [24]. What is more, a major recent meta-analysis of 40 studies involving over 135,000 participants demonstrated the marked benefits of EPA/DHA supplementation therapy in reducing major CVD outcomes [25].
Clinical evidence in the context of muscle wasting
The 2016 ESC guidelines [26] for the management of HF provided a general recommendation that “an n-3 PUFA preparation may be considered in symptomatic HF patients to reduce the risk of cardiovascular hospitalization and cardiovascular death” irrespective of the presence of sarcopenia (class of recommendation IIb, level of evidence B). Preference should be given to “n-3 PUFA preparations containing 850–882 mg of EPA and DHA as ethyl esters in the average ratio of 1 : 1.2”, with the recommendation based on the results of the GISSI-HF (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico – heart failure) trial [27]. No recommendation for the use of PUFA can be found in the 2021 version of the ESC guidelines [1]. In 2019, Rossato et al. reviewed the available evidence of omega-3 fatty acids intake/supplementation on muscle/lean mass (LM) and physical function in young and older adults. Observational studies have not demonstrated significant associations between omega-3 intake and muscle mass. The associations between PUFA intake and muscle function were not significant after adjustments for confounders, except for the observational study (with a longitudinal design) that showed ALA to be associated with knee extension strength [21, 23]. The evidence on the effects of omega-3 supplementation on muscle mass in sedentary young and older adults is also rather mixed. There is also conflicting evidence whether supplementation confers a beneficial effect on muscle function in older adults [19]. A meta-analysis of 10 randomized controlled trials published in 2020 evaluated the potential effects of omega-3 fatty acid supplementation on sarcopenia-related performance among the elderly [28]. The authors included RCTs that evaluated the effect of increasing n-3 PUFAs (through diet or supplementation) on skeletal muscle mass, muscle strength, or muscle performance in adults aged 60 years or older. A total of 552 participants were included. There were small benefits for muscle mass gain (0.33 kg; 95% confidence interval (CI): 0.05, 0.62) and timed up and go performance (–0.30 s; 95% CI: –0.43, –0.17). Subgroup analyses regarding muscle mass and walking speed indicated that omega-3 fatty acid supplements at > 2 g/day may contribute to muscle mass gain (0.67 kg; 95% CI: 0.16, 1.18) and improve walking speed, especially among those who received the intervention for > 6 months (1.78 m/s; 95% CI: 1.38, 2.17). The authors concluded that the appropriate supplementation of n-3 PUFAs may have benefits on muscle mass and performances among the elderly, however these results still need to be confirmed in the context of clinical relevance [28].
Studies in heart failure muscle wasting
Only one randomized trial has been undertaken in patients with HF. Mehra et al. conducted a small, randomized, double-blind, placebo-controlled trial in 14 patients with symptomatic HF, New York Heart Association (NYHA) class III–IV. Seven patients were given 8 g of n-3 fatty acids (group A) or placebo (group B) daily for 18 weeks. The study showed that 8 g PUFA intake daily over 18 weeks was associated with a significant reduction of TNF production in lipopolysaccharide-stimulated peripheral blood mononuclear cells in vitro. This intervention was also associated with a trend towards an increase in body fat mass (p = 0.07), but body weight and muscle mass did not change. It is worth emphasizing that there were no safety concerns in relation to such a high dose of PUFA, which was well tolerated. However, it is not known whether the changes in TNF concentrations in vitro are clinically relevant [29].
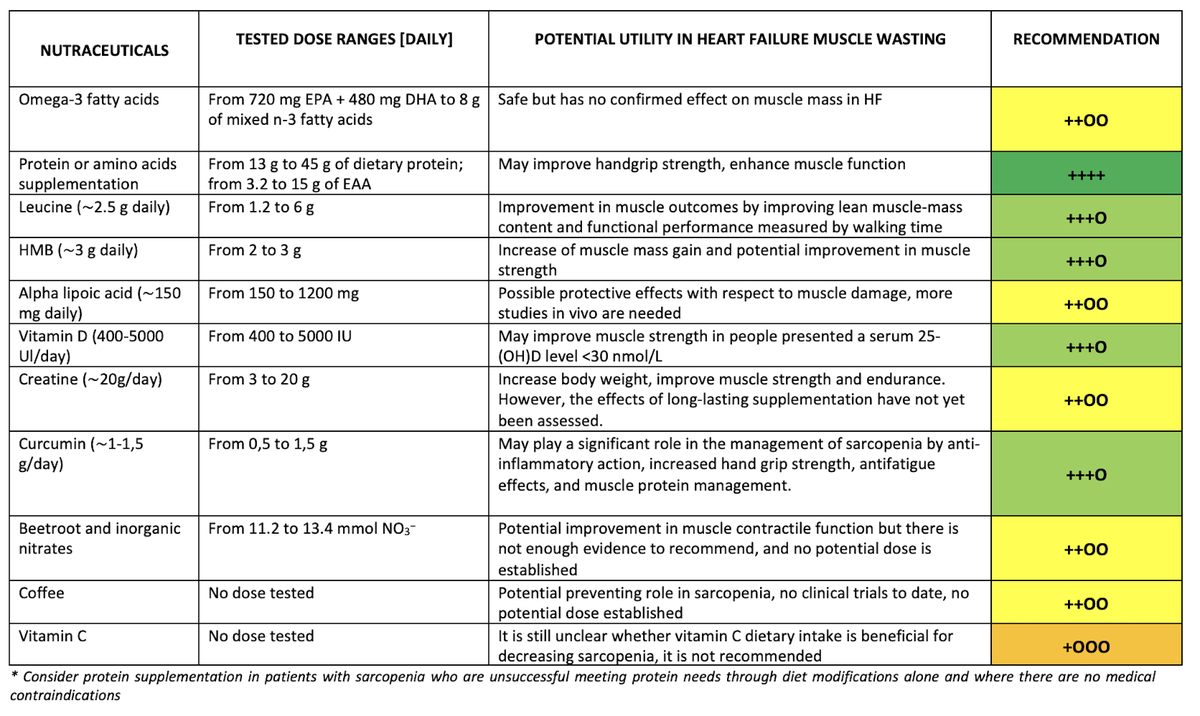
A summary of potential utility of omega-3 fatty acids in heart failure muscle wasting along with ILEP recommendations is available in Table II.
Table II
Recommendations on potential utility of selected nutraceuticals in heart failure muscle wasting
| Nutraceutical | Tested dose ranges [daily] | Potential utility in heart failure muscle wasting | Recommendation | References |
|---|---|---|---|---|
| Omega-3 fatty acids | From 720 mg EPA + 480 mg DHA to 8 g of mixed n-3 fatty acids | Safe but has no confirmed effect on muscle mass in HF | ++OO | 19, 29 |
| Protein or amino acids supplementation | From 13 g to 45 g of dietary protein; from 3.2 to 15 g of EAA | May improve hand grip strength, enhance muscle function | ++++ | 45, 46, 47, 48, 55, 56, 57, 84, 160 |
| Leucine (∼2.5 g daily) | From 1.2 to 6 g | Improvement in muscle outcomes by improving lean muscle-mass content and functional performance measured by walking time | +++O | 37, 42, 49, 50, 54 |
| HMB (∼3 g daily) | From 2 to 3 g | Increase of muscle mass gain and potential improvement in muscle strength | +++O | 37, 38, 39, 40, 41, 51, 52, 53 |
| Alpha lipoic acid (∼150 mg daily) | From 150 to 1200 mg | Possible protective effects with respect to muscle damage, more studies in vivo are needed | ++OO | 59, 68, 69 |
| Vitamin D (400–5000 UI/day) | From 400 to 5000 IU | May improve muscle strength in people presenting a serum 25-(OH)D level < 30 nmol/l | +++O | 78, 79, 80, 81, 82, 83, 84, 87 |
| Creatine (∼20 g/day) | From 3 to 20 g | Increase body weight, improve muscle strength and endurance. However, the effects of long-lasting supplementation have not yet been assessed | ++OO | 96, 99, 115, 116, 117, 118 |
| Curcumin (∼1–1.5 g/day) | From 0.5 to 1.5 g | May play a significant role in the management of sarcopenia by anti-inflammatory action, increased hand grip strength, antifatigue effects, and muscle protein management | +++O | 127, 128, 129 |
| Beetroot and inorganic nitrates | From 11.2 to 13.4 mmol NO3– | Potential improvement in muscle contractile function but there is not enough evidence to recommend, and no potential dose is established | ++OO | 18, 131 |
| Coffee | No dose tested | Potential preventing role in sarcopenia, no clinical trials to date, no potential dose established | ++OO | 143, 149 |
| Vitamin C | No dose tested | It is still unclear whether vitamin C dietary intake is beneficial for decreasing sarcopenia, it is not recommended | +OOO | 153, 154, 155, 157 |
Essential amino acids/leucine
Despite the fact that amino acids are not typical natural products, we have decided to include them in this Position Paper, due to their potentially positive role in patients with HF muscle wasting. Essential amino acids (EAA) are amino acids that humans and other vertebrates cannot synthesize from metabolic intermediates [30]. EAA oral supplementation has anabolic effects, enhances protein synthesis in muscles, and inhibits proteolysis, an effect that seems to be most pronounced for leucine, a branched-chain amino acid (BCAAs) [3]. BCAAs, namely leucine, isoleucine and valine, are essential amino acids with interesting and clinically relevant metabolic effects [31]. BCAAs have been suggested as a useful supplementation in the treatment of cachexia and reduced muscle mass as they may exert anabolic effects by promoting protein synthesis and by inhibiting proteolysis [32]. Several animal studies have demonstrated that BCAAs, particularly leucine, exert significant effects on skeletal muscle protein such as stimulation of muscle protein synthesis also by possible interactions with the innate immune system with resulting modified cytokine expression [33]. Leucine is thought to facilitate insulin signalling and glucose uptake in skeletal muscle cells through the PI3K-AKT-mTOR pathway [34]. Leucine supplementation appears to modulate inflammatory status and protein turnover of muscle cells by altering protein synthesis and protein degradation pathways. In the animal study by Papineau et al. leucine supplementation decreased markers of inflammation at the onset of skeletal muscle regeneration, potentially leading to an improved skeletal muscle regenerative response [35].
In a cross-over, double-blinded design, 8 healthy men were randomly assigned to one of three experimental conditions: supplementation with BCAAs or LEU in comparison with isonitrogenous placebo (PLA; 2.4 g of leucine, 1.6 g of isoleucine, and 1.6 g of valine) versus LEU (2.4 g of leucine + 3.2 g of alanine) versus alanine (5.6 g) as placebo. No significant differences were found in serum TNF levels 30 min after supplement intake. However, serum IL-6 and IL-10 concentrations significantly decreased 60 min after LEU supplementation when compared to the placebo intervention (p < 0.05) [36]. A leucine metabolite, β-hydroxy β-methylbutyrate (HMB), is of special relevance as it is thought to exert its effects through protective, anticatabolic mechanisms and has been shown to directly influence protein synthesis and mitochondrial dynamics in skeletal muscle [37]. The results of the study by Gepner et al. indicated that 40 days of HMB supplementation (3 g daily) attenuate inflammatory status by changes in circulating TNF [F-F ratio] (F = 6.48, p = 0.006), CX3CL1 (F = 4.70, p = 0.025), IL-1β (F = 6.93, p = 0.006), IL-2 (F = 4.96, p = 0.019), IL-6 (F = 6.27, p = 0.012) and IL-10 (F = 3.72, p = 0.041) plasma concentrations during highly intense military training [38]. The attenuation of the cytokine response to highly intense exercise protocols when supplementing with HMB was also reported in previous studies [39–41]. Jakubowski et al. reported that in young men undertaking an undulating periodised resistance training program, the ingestion of whey protein (50 g) with HMB (3 g daily) versus whey protein with the same amount of leucine (3 g daily) resulted in no differences in training-induced gains in lean body mass, muscle size, strength, or power [42].
Clinical evidence in the context of muscle wasting
Proteins and amino acids are the most studied types of dietary supplements to improve muscle outcomes [43]. In 2013 the PROT-AGE Study Group published the Evidence-based recommendations for optimal dietary protein intake in older people [44]. Experts recommended that to maintain physical function, older people need more dietary protein than do younger people (average daily intake at least in the range of 1.0 to 1.2 g/kg body weight/day). Most older adults who have an acute or chronic disease need even more dietary protein (i.e., 1.2–1.5 g/kg body weight /d); people with severe illness or injury may need as much as 2.0 g/kg body weight/day [44]. In the systematic review prepared to evaluate the clinical evidence reporting the effect of nutrition in sarcopenia, diagnosed according to the definition proposed by the European Working Group on Sarcopenia in Older People, 11 of the 12 included studies contained EAAs, HMB or protein [45]. These findings, consistent with the observational evidence of differences in habitual protein intake, showed the effects of supplementation seem to be mixed [45]. More recent systematic reviews of protein/amino acid supplementation in older adults have yielded similar findings [46–48]. Although some evidence of benefit was found in individual studies of older adults, pooled analyses have shown that the overall effects on muscle strength were not significant [46]. In other meta-analyses in the group of non-frail community-dwelling older adults the authors showed that while there was a tendency for grip strength to increase in protein-supplemented participants (standardized mean difference [SMD]: 0.58; 95% CI: –0.08–1.24, p = 0.08), there were no differences in lower extremity strength [47]. On the other hand, the most recent meta-analysis of 32 RCTs of nutritional supplementation in older adults by Veronese et al. indicated that protein or amino acids supplementation significantly improved hand grip strength (SMD = 0.24, 95% CI: 0.07–0.41) [48].
A systematic review published in 2019 by Martínez-Arnau et al. analysed the effects of leucine supplementation in older individuals with sarcopenia [49]. The authors showed that administration of leucine or leucine-enriched proteins (at the doses of 1.2–6 g leucine/day) is well-tolerated and significantly improves sarcopenia in older individuals, mainly by improving lean muscle-mass content. Mixed results were reported regarding the effect of supplementation on muscular strength, and there are still limited data on the effect on physical performance. For sarcopenia-associated individuals with concomitant disorders, the most promising effects of leucine supplementation are reported for the rehabilitation of post-stroke patients and in those with liver cirrhosis [49]. In 2020 the same author conducted a placebo-controlled, randomized, double-blind study with 50 participants of both sexes aged 65 years and over living in nursing homes. All individuals fulfilled the inclusion criterion to be able to walk 6 m and were 65 years or over. The participants were randomized to a parallel group intervention of 13 weeks’ follow-up with an intake of leucine at the dose of 6 g/day or placebo (lactose, 6 g/day). Administration of leucine was well-tolerated and significantly improved some criteria of sarcopenia in older individuals, such as functional performance measured by walking time (p = 0.011), and also improved lean mass index [50].
Multiple studies on HMB supplementation have been conducted, with several available systematic reviews and meta-analyses evaluating its effects, either alone or in combination with other amino acids, on muscle quality and function both in older adults with sarcopenia and other pathological conditions. Some studies with HMB have shown to improve muscle mass and to preserve muscle strength and function in older people with sarcopenia or frailty [37]. The study by Wu et al. aimed to investigate whether HMB supplementation had significant effects on body composition and muscle strength in healthy older adults and those with pathological conditions at the age of ≥ 65 years [51]. The meta-analysis of 6 studies showed that HMB supplementation alone or in combination with other amino acids, increased muscle mass gain in the intervention groups compared with the control groups (SMD 0.352 kg; 95% CI: 0.110–0.594; Z value = 2.85; p = 0.004). There were, however, no differences with regard to changes in fat mass between groups (SMD = –0.08 kg, p = 0.511) [51]. Bear et al. investigated the efficacy of HMB alone, or supplements containing HMB, on skeletal muscle mass and physical function in a variety of clinical conditions characterized by loss of skeletal muscle mass and weakness. In the meta-analysis of 15 RCTs with 2137 patients the authors revealed some evidence to support the effect of HMB alone, or supplements containing HMB, on increasing skeletal muscle mass (SMD = 0.25; 95% CI: –0.00, 0.50; z = 1.93; p = 0.05; I2 = 58%) and strong evidence has been provided to support improvements in muscle strength (SMD = 0.31; 95% CI: 0.12–0.50; z = 3.25; p = 0.001; I2 = 0%) [52]. Courel-Ibáñez et al. aimed to quantify the effects of exercise in addition to HMB supplementation, on physical and cognitive health in older adults. Based on the data from 10 RCTs with 384 participants, investigating the effect of HMB supplementation and physical function in adults aged ≥ 50 years, they showed that HMB supplementation, in addition to physical exercise, has no or relatively low impact on improving body composition, muscle strength, or physical performance compared with exercise alone [53]. The umbrella review published in 2021 on behalf of the Sarcopenia Guidelines Development Group of the Belgian Society of Gerontology and Geriatrics included systematic reviews, which examined the effect of essential amino acids and leucine supplementation on improving muscle mass or strength in older people. No convincing evidence was found to support supplementation with essential amino acids. The authors concluded that EAAs supplementation should not be considered as an intervention to increase muscle mass, muscle strength, and physical performance. Nevertheless, the umbrella review provided sufficient evidence to recommend leucine supplementation for sarcopenic older people to increase muscle mass, but not for muscle strength or physical performance [54].
Studies in heart failure muscle wasting
Supplementation studies have been conducted in patients with HF. An investigator-blinded, randomized study by Aquilani et al. included HF patients and showed that peak oxygen consumption and 6min walking distance test was improved within 2 months of oral supplementation with a mix of all EAAs [55]. A similar RCT of oral amino acid supplementation in older patients with chronic HF showed a small improvement in peak oxygen consumption at 30 days [56]. However, a more recent open label, randomized, controlled study failed to confirm these results. The study evaluated combined therapy with resistance exercise training and BCAA supplementation in 66 patients with chronic HF. Supplementation with essential amino acids did not provide benefit beyond resistance exercise training alone [57]. According to the 2021 HF guidelines of the European Society of Cardiology (ESC), the most effective strategy for sarcopenia treatment is resistance exercise training, possibly combined with a protein intake of 1–1.5 g/kg/day [1].
Nutritional studies in sarcopenia researched the effects of protein as well as amino acids supplementation in form of EAA supplements, leucine supplements or supplements with leucine metabolite-HMB. Several systematic reviews and meta-analyses aimed to examine and compare the effects of diverse nutritional interventions [45–48, 54]. Therefore, we decided to describe all available evidence in one section of our statement. However, in Table II we summarized a potential utility of analysed supplements in heart failure muscle wasting in 3 categories: 1) protein or amino acids supplementation; 2) leucine; and 3) HMB. ILEP recommendations are also available in Table II.
Alpha lipoic acid (LA)
Alpha lipoic acid (LA) is part of the multienzyme complex of pyruvate dehydrogenase, alpha-ketoglutarate and branched alpha-keto acids [58, 59]. Additionally, LA has antioxidative effects via the ability to recycle endogenous glutathione and via acting as a radical scavenger of hydroxy radicals, hypochlorous acids, peroxide radicals and singular oxygen, as well as by forming chelate complexes with metal ions [60–63]. Anti-inflammatory effects of LA have also been described. LA inhibits the transduction of the nuclear factor kB (NF-kB) by modulating mitogen-activated protein kinase (MAPK) via inhibitor kB (IkB) reducing inflammation [64, 65]. LA decreased levels of C-reactive protein (CRP), TNF, interleukin (IL) 6, IL-8 and IL-10 in patients with type 2 diabetes as well as in patients with metabolic syndrome [66, 67].
Clinical evidence in the context of muscle wasting
A study by Morawin et al. demonstrated that LA reduces serum concentrations of creatine kinase after a 90-min endurance load indicating possible protective effects with respect to muscle damage [59]. Isenmann et al. conducted a double-blind, randomised, controlled trial which enrolled 17 male athletes experienced in resistance and endurance exercise. A moderate inhibition of muscle damage and inflammation was observed in the LA-group compared to placebo [68]. Di Cesare Mannelli et al. showed synergistic effects of the R(+) stereoisomer of lipoic acid (R(+)LA) and β-hydroxy-β-methyl butyrate (HMB) against dexamethasone (DEX)-dependent damage of myoblast- and myotube-cell cultures. The combination of R(+)LA with HMB was the only treatment able to significantly reduce DEX-dependent redox imbalance, by reducing the concentration of O2– and the level of carbonylated proteins, an oxidative modification due to the introduction of carbonyl groups into protein side chains leading to decreased functionality [69].
A summary of the potential utility of alpha lipoic acid in heart failure muscle wasting along with ILEP recommendations is available in Table II.
Vitamin D
Vitamin D is a fat-soluble vitamin that can act as a hormone through a nuclear receptor [70]. The two major biologically inert precursors of vitamin D are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). Humans obtain vitamin D through dietary intake and exposure to sunlight [71]. According to the Endocrine Society’s Practice Guidelines, vitamin D deficiency was defined as a serum 25(OH)D < 20 ng/ml, insufficiency as 21–29 ng/ml and sufficiency as at least 30 ng/mL for maximum musculoskeletal health [72]. Vitamin D deficiency and insufficiency is a global health problem, it has been estimated that 20–100% of U.S., Canadian, and European older men and women living in the community are vitamin D deficient [72]. The most important function of vitamin D is in the regulation of Ca2+ concentration in circulating blood, which deficiency may lead to diseases such as rickets in children and osteomalacia in adults. It has recently been proven that deficiency or insufficiency in vitamin D may be positively associated with the risk of several diseases including sarcopenia, cardiovascular diseases, obesity, and cancer [70]. Furthermore, there is an association of vitamin D status with biomarkers of oxidative stress and inflammation such as CRP, IL-6, cathepsin S, vascular cell adhesion molecule-1 (VCAM-1), malondialdehyde (MDA), myeloperoxidase, 3-nitrotyrosine, and superoxide dismutase (SOD) [73]. The observation that in vitro vitamin D3 is effective in stabilizing endothelial membranes thereby reducing inflammation may help explain clinical observations of extremely high doses of vitamin D being effective in treating or reducing symptoms of some autoimmune disorders like psoriasis, vitiligo, and multiple sclerosis [74–76]. The biological, epidemiological and clinical evidence supporting the association between vitamin D and an increased risk of sarcopenia in older people have been reviewed by Remelli et al. [77].
Clinical evidence in the context of muscle wasting
Visser et al. conducted a prospective, population-based study, which showed that a lower 25-(OH)D circulating concentration and a higher parathyroid hormone (PTH) concentration increased the risk of sarcopenia (loss of hand grip strength and loss of appendicular skeletal muscle mass) in old age [78]. Bunout et al. assessed the effects of resistance training and vitamin D supplementation on physical performance of healthy older subjects. The study showed that gait speed was higher among subjects supplemented with vitamin D (whether trained or not) than in non-supplemented subjects (838 ±147 and 768 ±127 m/12 min, respectively, p = 0.02) [79]. Wicherts et al. investigated the association of serum 25-(OH)D concentration with current physical performance and its decline over 3 years among older subjects. The study showed that serum 25-(OH)D concentrations below 20 ng/ml were associated with poorer physical performance and a greater decline in physical performance in older men and women [80]. A systematic review and meta-analysis of 30 RCTs with 5615 individuals (mean age: 61 years) on the effects of vitamin D on skeletal muscle strength, muscle mass and muscle power revealed a small but significant positive effect of vitamin D supplementation on global muscle strength with SMD of 0.17 (p = 0.02). No significant effect was found on muscle mass (SMD = 0.058; p = 0.52) or muscle power (SMD = 0.057; p = 0.657). Effects on muscle strength were significantly greater in individuals who presented a baseline serum 25-(OH)D level of < 30 nmol/l [81]. A 2015 meta-analysis of seven controlled trials with vitamin D supplementation showed a significant improvement in upper and lower limb muscle strength in healthy 18- to 40-year-old participants [82]. Insufficient evidence was found to support the use of vitamin D supplementation specifically for sarcopenia, although there is evidence that individuals with low vitamin D levels may improve their strength with vitamin D supplementation [83]. A meta-analysis by Gkekas et al. based on 8 studies (776 patients) aimed to evaluate the effect of vitamin D alone, or with protein supplementation, on muscle strength, mass, and performance in sarcopenia. Vitamin D at the doses of 100–1600 IU/day plus protein (10–44 g/day) supplementation showed a beneficial effect on muscle strength, as demonstrated by an improvement in hand grip strength (SMD 0.38 ±0.07, 95% CI: 0.18–0.47, p = 0.04) and a decrease in the sit-to-stand time (SMD 0.25 ±0.09, 95% CI: 0.06–0.43, p = 0.007) in comparison to the placebo group [84]. The effect on muscle mass, measured by skeletal muscle index, was marginally non-significant (SMD 0.25 ±0.13, 95% CI –0.006–0.51, p = 0.05), and no significant effect on appendicular skeletal muscle mass or muscle performance (measured by walking speed) was observed [84].
Studies in heart failure muscle wasting
The largest trial of vitamin D supplementation recruited 400 patients with advanced HF and randomized them to 4000 I.U. vitamin D3 daily or matching placebo in a 1 : 1 ratio [85]. Even though the authors did not find a difference in the primary endpoint (all-cause mortality), they noted a significant improvement in left ventricular performance over 3 years of follow-up in the subgroup of patients aged ≥ 50 years [increase in LVEF of 2.73% (95% CI: 0.14–5.31%) at 12 months post-randomization and 2.60% (95% CI: –2.47 to 7.67%) at 36 months post-randomization] [86]. Unfortunately, skeletal muscle function mass and function were not assessed during the trial. Patients in the vitamin D supplementation group had a greater need for mechanical circulatory support implantation, particularly when baseline serum levels of 25-OH-D were ≥ 30 nmol/l, prompting the authors of the study to suggest caution in the long-term supplementation of vitamin D in this clinical setting. Only one study has investigated muscular performance in patients with HF. Boxer et al. designed a cross-sectional study to identify relationships between anabolic hormones, inflammatory markers and physical function in patients with HF. Sixty patients (43 men and 17 women) with an ejection fraction of 40% or less were included [87]. The authors performed a 6-minute walk test (6-MWT), measured a frailty phenotype, and performed multiple biochemical tests. The study showed that longer 6-MWT distance was correlated with higher serum 25-hydroxyvitamin D (25-OH-D) level (correlation coefficient: 0.42; p < 0.05). A shorter walk was correlated with higher cortisol: dehydroepiandrosterone sulphate (DHEAS) ratio, high-sensitivity CRP, IL-6, and intact PTH (correlation coefficients: –0.26; –0.50; –0.46; –0.31 respectively, all p < 0.05). Higher frailty phenotype score was correlated with higher high-sensitivity CRP, higher IL-6, and lower serum 25(OH)D levels (correlation coefficients: 0.32; 0.32; –0.30 respectively, all p < 0.05). Therefore, taken together, lower vitamin D levels and higher CRP levels were associated with poor aerobic capacity and greater frailty, whereas no significant role for other hormones such as testosterone, dehydroepiandrosterone (DHEA), or cortisol was found. The authors concluded that further studies are required to examine whether altering vitamin D intake and inflammation status in patients with HF will improve functional and aerobic capacity [87].
A summary of potential utility of vitamin D in heart failure muscle wasting along with ILEP recommendations is available in Table II.
Creatine
Creatine (N-aminoiminomethyl-N-methyl glycine) is a naturally occurring and nitrogen-containing compound comprised of amino acids [88]. Creatine is mostly (95%) stored in muscle, where it plays a critical role in providing energy through the creatine kinase (CK) and phosphocreatine (PCr) system. Endogenous creatine synthesis provides about half of the daily requirements of this compound. The remaining amount of creatine needed to maintain normal tissue levels is obtained from diet, primarily from red meat and fish or dietary supplements. A normal-sized individual needs to consume 2–3 g/day of creatine to maintain normal stores depending on diet, muscle mass, and physical activity levels. The role of creatine in energy metabolism in diseases depends on the CK/PCr system, which provides the metabolic basis on how creatine can affect health and how it can offer therapeutic benefit [88].
Creatine has been shown to act as an antioxidant and, as such, may reduce indices of inflammation in aging adults [89]. Creatine supplementation may also play a role in downregulating oxidative stress associated with inflammation [89, 90]. In an in vitro study, creatine (0.5–5 mM) suppressed the adhesion of neutrophils to endothelial cells and inhibited the binding of intracellular adhesion molecule-1 (ICAM-1) and E-selectin, suggesting its anti-inflammatory effect [91]. Furthermore, creatine may attenuate the increase in a pro-inflammatory immune system response to aerobic exercise [92–94], but appears to provide no inflammatory benefit for resistance-trained individuals [95]. However, HF patients who were administered creatine (5 g/day) and performed aerobic exercise (3 days per week) experienced a significant reduction in systemic IL-6, CRP, and endothelial function (ICAM-1, P-selectin) compared with the control group [96, 97].
Clinical evidence in the context of muscle wasting
Creatine is one of the most popular nutritional ergogenic aids for athletes [98]. Many available studies have shown that creatine supplementation increases intramuscular creatine concentrations, can improve exercise performance, and/or improve training adaptations [98]. For older adults, several studies have demonstrated that creatine monohydrate supplementation in addition to a strength training protocol can augment muscle mass and function [99, 100]. It has been shown that this might be due to energy and mechanical optimization of the cells, which results in the prevention of protein degradation, an activation of satellite cells and an increase in glycogen synthesis [101]. Three meta-analyses have been performed to determine the efficacy of creatine (≥ 3 g/day) vs. placebo during a resistance training program (≥ 7 weeks) on measures of muscle accretion and strength [102–104]. Collectively, these meta-analyses showed that the combination of creatine and resistance training augmented muscle accretion (≈1.2 kg), and upper- and lower-body strength, more than placebo and resistance training alone in older adults [99]. Research regarding the effectiveness of creatine supplementation without resistance training is mixed with 5 studies showing greater effects from creatine vs. placebo [105–109] and 5 studies showing similar effects between the two interventions [110–114]. The majority of studies that reported beneficial effects from creatine incorporated a creatine loading phase (20 g/day) or used a high relative daily dosage of the substance (0.3 g/kg/day), whereas several of the studies that failed to observe beneficial effects did not use these strategies [99, 115].
Studies in heart failure muscle wasting
Studies have been conducted evaluating creatine supplementation in patients with HF. Gordon et al. conducted a double-blind, placebo-controlled study in 17 patients with a left ventricular ejection fraction < 40%. All participants were supplemented with creatine 20 g daily for 10 days. The authors found that in the study group, creatine supplementation improved muscle strength and endurance [116]. Andrews et al. conducted a study in which 20 patients with chronic HF were randomly allocated in a double-blind fashion to receive 5 g of creatine four times a day or matching placebo for 5 days. The study showed that creatine increased muscle endurance (defined as the number of contractions until exhaustion at 75% of maximum voluntary strength) and reduced lactate and ammonia production under the same conditions. The authors concluded that creatine supplementation in chronic HF augments skeletal muscle endurance and attenuates the abnormal skeletal muscle metabolic response to exercise [117]. Finally, Kuethe et al. designed a double-blind, placebo-controlled study involving 20 patients with congestive HF for more than 6 months and with a peak oxygen uptake (peak VO2) below 20 ml/min/kg. The study group received 5 g of creatine four times a day for 6 weeks, while the control group received placebo. After 6 weeks of creatine supplementation there was a significant increase in body weight and muscle strength compared with baseline and placebo (p < 0.05). Therefore, the study showed that short-term creatine supplementation in addition to standard medication in patients with congestive HF can increase body weight and improve muscle strength [118].
A summary of potential utility of creatine in heart failure muscle wasting along with ILEP recommendations is available in Table II.
Curcumin
Curcumin comes from the root of turmeric of curcuma (an herbaceous perennial of the Zingiberaceae), includes about 2–8% turmeric and is commonly consumed daily throughout Asian countries without reported toxicity [119, 120]. Curcumin has been reported to possess diverse pharmacological effects including antioxidant and anti-inflammatory activities, it may also inhibit PCSK9 protein and have some anti-atherosclerotic properties [119, 121]. The effects of curcumin on muscle wasting have not been fully established. In a study conducted by Lee et al., spray dry powder containing 40% curcumin (CM-SD) demonstrated utility against oxidative stress and inflammation-related muscle disorders by transactivation of the nuclear factor erythroid-2-related factor 2 (Nrf2)-dependent luciferase activity, enhancement of the levels of heme oxygenase (HO)-1, glutamate cysteine ligase catalytic subunit (GCLC), and NAD(P)H-dependent quinone oxidoreductase (NQO)-1, as well as reduction of reactive oxygen species (ROS) production and lipid peroxidation and restored glutathione (GSH) depletion in H2O2-treated C2C12 cells [122]. Inhibition of the NF-kB pathway by curcumin, which is a well-established effect of this phytochemical, could justify the potential benefit of curcumin supplementation to attenuate muscular atrophy in catabolic conditions [123].
In the International Lipid Expert Panel position paper on impact of nutraceuticals on markers of systemic inflammation a Class IIa Level B recommendation was made for curcumin [23]. The authors stressed that in the recent meta-analysis of 15 RCTs, which was conducted to assess the influence of curcumin-containing supplements on biomarkers of inflammation and oxidative stress, a significant reduction in circulating IL-6 and CRP levels was reported [124].
Clinical evidence in the context of muscle wasting
Curcumin treatment successfully prevented the development of pre-sarcopenia and sarcopenia by improving satellite cell commitment and recruitment in mice after 6 months of treatment [125]. Based on a study by Tanabe et al., ingestion of curcumin before exercise may attenuate acute inflammation, and after exercise it could attenuate muscle damage and facilitate faster recovery [126]. In a human study by Gorza et al., 19 men were randomized to examine the effects of curcumin supplementation at the dose of 1.5 g/day compared with a placebo (PLA) following a muscle-damaging protocol (MDP) on oxidative stress, inflammation, muscle damage, and soreness. The MDP was performed before and 28 days after supplementation. Blood was sampled pre- and at 60 min, 24 h, and 48 h post exercise and analysed for total antioxidant capacity (TAC), malondialdehyde (MDA), TNF, and CK. After supplementation, curcumin significantly reduced CK levels (199.62 U/l) compared to the placebo (287.03 U/l; p < 0.0001). Curcumin supplementation also resulted in decreased muscle soreness (visual analogue scale (VAS) 2.88), when compared with placebo (VAS 3.36, p = 0.012). No differences were observed in TAC, TNF, or MDA [127]. In a study by Hillman et al. the authors aimed to determine the effects of curcumin supplementation on delayed onset muscle soreness and muscle power following plyometric exercise. 39 participants consumed either curcumin (500 mg) or placebo twice daily for 10 days (6 days pre-exercise, on the day of exercise and for the 3 days following exercise); on day 7 all participants completed 5 × 20 drop jumps. Soreness was greater in placebo vs. curcumin 48 h and 72 h post-exercise (p < 0.01); however, CK was not significantly different between groups (p = 0.28). Vertical jump (countermovement jump was used to assess muscle power) decreased over time in the placebo, but not in the curcumin group (19.8 ±4.8 vs. 21.4 ±3.2; p = 0.01). The authors suggest that curcumin reduces soreness and maintains muscular power following plyometric exercise [128]. A randomized, placebo-controlled, double-blind study involving 30 subjects evaluated the efficacy of curcumin supplementation in the management of sarcopenia conditions. Curcumin supplementation was associated with a significant increase by 1.43% (p < 0.001) in hand grip strength compared with placebo. The weight-lifting capacity also showed an increase of 6.08%, whereas in the placebo group a 4.54% decrease at the end of the study period was observed. The results confirmed that curcumin tended to have a positive impact on distance covered before feeling tired as shown by an increase (p = 0.09) of 5.51%, compared with the placebo group, which showed an increase of only 2.29%. The time taken to walk the same distance was reduced in the curcumin group (1.15%), whereas in the placebo group it was increased (2.02%) [129].
A summary of potential utility of curcumin in heart failure muscle wasting along with ILEP recommendations is available in Table II.
Beetroot and inorganic nitrates
In mammals, including humans, nitric oxide (NO) serves as a signalling molecule involved in several physiological and pathological processes [130]. Within the cardiovascular system, basal endothelial NO release plays a critical role in sustaining cardiovascular health and it does this in many ways including its role as a vasodilator, anti-proliferative, anti-leucocyte and anti-platelet agent [130]. In skeletal muscle, NO helps to modulate contractile function, through the nitrosation or S-nitrosylation of various proteins. Furthermore, during concentric activity, NO significantly increases the rate of force development, maximal power and maximal shortening velocity of both single muscle fibres and isolated muscles, via the activation of the classic NO soluble guanyl cyclase-cyclic GMP (NO-sGC-cGMP) pathway [18]. In patients with HF, an increased production of reactive oxygen species leads to a decline in the bioavailability of NO and decreased NO-sGC-cGMP signalling [131]. The orally available activator of soluble guanylate cyclase, vericiguat, has been shown to exert beneficial effects in a recent large-scale randomized trial [132]. It is possible that NO bioavailability is diminished in skeletal muscles of patients with HF, thus contributing to their reduced muscle function. Furthermore, HF leads to endothelial dysfunction in various tissues, including skeletal muscles, as a result of reduced NO production by endothelial NO synthase [18]. The primary source of NO in humans is NO production from L-arginine by enzymes called nitric oxide synthases (NOS) [133]. Nevertheless, NO is also derived from dietary nitrate (NO3–). In this alternative pathway, NO3– is first reduced to nitrite (NO2–) by oral facultative anaerobic bacteria. After NO2– is swallowed, acidic conditions in the stomach and/or other tissues (e.g., contracting muscle) can further reduce it to NO [131]. There is emerging evidence, which suggests that supplementation of dietary inorganic nitrate (NO3–) has beneficial effects on vascular health, blood pressure, exercise capacity and oxygen metabolism through targeted NO production [18]. Beetroot juice is a rich natural source of NO3– and could be used as nutraceutical [134]. Indeed, the anti-inflammatory effects of red beetroot have been demonstrated in several studies. It has been reported that treatment of rats with the ethanol extract of red beetroot for 28 days combats against the nephrotoxicity of gentamicin and reduces the activity of NF-kB, TNF, and IL-6 [135]. A study by Pietrzkowski et al. has shown that capsules with the betalain-rich red beet extract taken for 10 days decreased pre-inflammatory factors such as cytokines, TNF, IL-6 in osteoarthritis affected patients [136]. The proposed mechanism for anti-inflammatory properties of betalains seems to be via interference with NF-kB, which reduces inflammatory mediators, as well as phagocyte cells [137]. Betalains have been also shown to inhibit the lipoxygenase enzyme and ICAM-1 in vitro, which is expressed in response to cytokine stimulation [138].
Clinical evidence in the context of muscle wasting
Even though there were a large number of studies which have examined dietary nitrate in relation to the cardiovascular system, an effort has also been made to uncover the benefits of dietary nitrate on muscle contractility [139]. Justice et al. performed a small-scale pilot trial to assess the potential efficacy of sodium nitrite supplementation for improving multiple domains of motor function in healthy middle-aged and older adults (62 ±7 years). The study showed that 10 weeks of daily nitrite supplementation significantly improved the rate of torque development during voluntary knee flexion/extension [140]. Coggan et al. conducted a study which aimed to test if dietary nitrate supplementation could increase muscle contractile function in older subjects. Twelve healthy older men and women (mean age: 71 ±5 years) were studied using a randomized, double-blind, placebo-controlled, crossover design. After fasting overnight, subjects were tested 2 h after ingesting beetroot juice containing or devoid of 13.4 ±1.6 mmol NO3–. The study showed that after NO3– supplementation, maximal velocity of knee extension increased by 10.9 ±12.1% (p < 0.01) and maximal knee extensor power increased by 4.4±7.8% (p<0.05) [141]. Finally, Córdova-Martínez et al. evaluated the effect of supplementation with NO precursors (L-arginine and beetroot extract) on muscular function during a training period of 6 weeks in older men and women. The study was double-blind, placebo-controlled and involved 66 subjects randomly divided into 3 groups: placebo, arginine-supplementation, and beetroot extract-supplementation groups. At the end of the training period no significant effects were noticed with L-arginine or beetroot extract supplementation on endurance, strength, and Short Physical Performance Battery (SPPB) index. Nevertheless, beetroot extract supplementation improved physical fitness significantly (p < 0.05) in the sprint exercise in men (2.33 ±0.59 s) in comparison with baseline results (2.72 ±0.41 s) [142].
Studies in heart failure muscle wasting
One study has been conducted on the impact of dietary nitrates on muscle function in patients with HF [131]. Coggan et al. designed a double-blind, placebo-controlled, randomized crossover design study, in which authors determined the effects of dietary NO3– in 9 HF patients. All included subjects were diagnosed with systolic dysfunction and had an LVEF ≤ 45%. Each patient was studied twice using the same experimental protocol. On one occasion they were tested after ingesting 140 ml of a concentrated beetroot juice supplement containing 11.2 mmol of NO3–, and on the other after ingesting the same volume of NO3–-depleted beetroot juice. There was a 1–2-week washout period between treatments. Both subjects and investigators were blinded to the order of treatment. The study showed that ingestion of beetroot juice containing 11.2 mmol of NO3– resulted in a very large, ~20-fold increase (p < 0.0001) in plasma NO3– concentration. Plasma NO2– concentration did not significantly increase. On the other hand, whole-body NO bioavailability did increase significantly following dietary NO3–, as evidenced by a 35–50% (depending on the time of measurement) increase (p < 0.001–0.05) in breath NO over baseline. Dietary NO3– improved several measures of muscle contractile function. Beetroot juice increased peak knee extensor power by 9% (p = 0.07) and 11% (p < 0.05) at the two highest movement velocities tested (i.e., 4.71 and 6.28 rad/s). Maximal power (measured by fitting peak power data with a parabola) was therefore greater (i.e., 4.74 ±0.41 vs. 4.20 ±0.33 W/kg; p < 0.05) after dietary NO3– intake. Calculated maximal velocity of knee extension was also higher following NO3– ingestion (i.e., 12.48 ±0.95 vs. 11.11 ±0.53 radian/s; p < 0.05). Blood pressure was unchanged, and no adverse clinical events were observed. The authors concluded that the beetroot juice supplement was well-tolerated and markedly enhanced NO bioavailability in patients with systolic HF, which led to significant improvement in muscle contractile function [131].
A summary of potential utility of beetroot and inorganic nitrates in heart failure muscle wasting along with ILEP recommendations is available in Table II.
Coffee/caffeine
Coffee is a very popular beverage, second only to water in terms of total consumption worldwide [143]. The anti-oxidative and anti-inflammatory effects of coffee have been extensively reported. Thus, coffee has become a subject of interest for researchers interested in its potential health benefits. The available meta-analyses have shown that coffee intake is inversely associated with the incidence of chronic diseases, including cardiovascular disease, Parkinson’s disease, late-life cognitive decline, non-alcoholic fatty liver disease and cancer [143]. Caffeine present in coffee has other beneficial effects such as antioxidant and anti-inflammatory actions that are altering the cellular redox and inflammatory status in a dose-dependent manner [144].
Studies of the effect of coffee on skeletal muscle have shown that it can attenuate insulin resistance in high-fat diet-induced obese mice and enhance insulin-induced Akt phosphorylation in diabetic mice [145, 146]. Coffee polyphenols, such as caffeic acid and chlorogenic acid, have been shown to stimulate glucose transport by AMPK activation [147]. In a study by Guo et al. [148], the authors investigated the effects of coffee on skeletal muscle in an animal model using aged mice. They showed that in vivo coffee treatment attenuated the decrease in muscle weight and grip strength, increased the regenerating capacity of injured muscles, and reduced serum pro-inflammatory mediator levels in comparison to the control group. In vitro, using satellite cells isolated from aged mice, coffee treatment increased the cell proliferation rate, augmented the cell cycle, and increased the activation level of Akt intracellular signalling pathway compared with control mice. These findings may suggest that administration of coffee had a beneficial effect on age-related sarcopenia [148]. Jang et al. investigated the effect of coffee on skeletal muscle hypertrophy in a mouse model. Animals were fed a normal diet, or a normal diet supplemented with 0.3 or 1% coffee. Coffee supplementation was observed to increase skeletal muscle hypertrophy, while simultaneously upregulating protein expression of total MHC, MHC2A, and MHC2B in quadriceps muscle. Coffee also increased grip strength and PGC-1α (peroxisome proliferator-activated receptor gamma coactivator-1α) protein expression and decreased the expressions of TGF-β (transforming growth factor-β) and myostatin in triceps muscle. These results suggested that coffee increases skeletal muscle function and hypertrophy by regulating the TGF-β/myostatin-Akt-mTORC1 (mTORC1 = mammalian target of rapamycin complex 1) [143].
Clinical evidence in the context of muscle wasting
One epidemiological study investigated the effect of coffee on sarcopenia. Chung et al. analysed the association of coffee consumption and sarcopenia in older Korean men. The authors derived cross-sectional data from the 2008–2011 Korea National Health and Nutrition Examination Survey (KNHANES). The study of 1,781 men at the age at least 60 years showed that people who consumed at least 3 cups of coffee a day, compared with the group of individuals who drank less than one cup of coffee a day, showed significantly decreased sarcopenia development (adjusted odds ratio = 0.43; 95% CI: 0.20–0.94). The decrease, however, was not significant when the daily coffee consumption was 1 or 2 cups. In multivariate logistic regression models, significant associations were observed between sarcopenia and coffee consumption (p for trend = 0.039). The authors concluded that their data support the potential role of coffee in preventing sarcopenia [149]. Unfortunately, no clinical trials have evaluated the impact of coffee consumption on sarcopenia development, nor are there any published reports of clinical studies evaluating the usefulness of coffee consumption in patients with sarcopenic HF.
A summary of potential utility of coffee in heart failure muscle wasting along with ILEP recommendations is available in Table II.
Vitamin C
Ascorbic acid (vitamin C) protects against oxidative stress by reducing levels of free oxygen radicals and inhibiting oxidative cell damage and low-density lipoprotein (LDL) oxidation [150]. In addition, it improves arterial stiffness and immune function, and reduces inflammatory markers responsible for systemic inflammation [151]. Oxidative stress is one of the factors potentially implicated in the development of sarcopenia. Reactive oxygen species (ROS) can trigger atrophy and the loss of muscle function directly as well as upregulate inflammatory cytokine expression like that of TNF, IL-1 and IL-6. As a result, antioxidants have been proposed to combat the development of sarcopenia by inhibiting the generation of ROS. Vitamin C is considered as the most important hydrophilic antioxidant [152].
Clinical evidence in the context of muscle wasting
A few studies have been published on the question of whether dietary intake of vitamin C may be related to muscle strength or physical performance. In the New Mexico Aging Process Study, only women with a slower gait speed demonstrated a lower vitamin C intake, while men with a slower gait speed did not [153]. In the Hertfordshire Cohort Study, vitamin C intake was associated with faster chair-rise times and 3-m walk times in women, but not in men [154]. These studies appear to suggest a potential sexual dimorphism regarding antioxidant intake, especially with regards to vitamin C, muscle mass and physical performance. On the other hand, a study by Oh et al., which included 1433 subjects (658 men and 775 women) over the age of 60 years, failed to find an association between vitamin C and (pre)sarcopenia in either sex, as assessed by appendicular skeletal mass divided by weight being less than 1 SD below the mean of a reference sample [155]. Fingeret et al. assessed the cross-sectional and longitudinal association between self-reported vitamin C + E dietary supplementation and markers of grip strength and frailty in community-dwelling Swiss adults [156]. After 5.2-year follow-up, no associations were found between supplementation and change in grip strength for raw values and after multivariable adjustment when the authors took baseline vitamin C + E supplementation into account. The vitamins neither improved grip strength nor prevented low-grip strength over a 5-year period [156]. Finally, Cesari et al. conducted the InCHIANTI study (“Invecchiare in Chianti” study), which demonstrated that daily dietary intake of vitamin C was significantly associated with both knee extension strength and overall physical performance (based on walking speed, a chair-stand test, and a balance test) [157]. Nevertheless, it is still unclear whether dietary vitamin C intake is beneficial for influencing sarcopenia, and whether there is truly a sexually dimorphic effect [152].
A summary of potential utility of vitamin C in heart failure muscle wasting along with ILEP recommendations is available in Table II.
Limitations and cautions
There is relatively little evidence from RCTs measuring clinically relevant outcomes with regard to nutraceuticals and their usefulness as anti-inflammatory substances in patients with HF and sarcopenia. Medical professionals are encouraged to consider our recommendations when making decisions regarding the treatment of patients with muscle wasting and HF. However, the position paper does not override in any way the individual responsibility of healthcare professionals to make appropriate, accurate and patient-centred decisions, considering the patient’s medical history, and in consultation with the patient and/or, where appropriate, their guardian or caretaker.
Actual guidelines and future directions based on our recommendations
All available recommendations regarding nutraceutical support in muscle wasting were summarised in Table III. A summary of potential utility of all described nutraceuticals in heart failure muscle wasting along with ILEP recommendations is available in Table II. The authors of this Position Paper would like to strongly emphasize that presented nutraceuticals are not an alternative to pharmacotherapies but can be treated as an adjunctive support with traditional HF therapies because they may beneficially interfere with the hypermetabolic/inflammatory processes that are present in HF patients and influence the patients’ prognosis.
Table III
Available recommendations regarding nutraceutical support in muscle wasting
| Author | Year | Recommendations | Reference |
|---|---|---|---|
| PROT-AGE Study Group | 2013 | “To maintain physical function, older people need more dietary protein than do younger people; older people should consume an average daily intake at least in the range of 1.0 to 1.2 g/kg BW/d”. “Most older adults who have an acute or chronic disease need even more dietary protein (i.e., 1.2–1.5 g/kg BW/d); people with severe illness or injury may need as much as 2.0 g/kg BW/d”. “Older people with severe kidney disease who are not on dialysis (i.e. estimated GFR < 30 ml/min/1.73 m2) are an exception to the high-protein rule; these individuals need to limit protein intake”. “Protein quality, timing of intake, and amino acid supplementation may be considered so as to achieve the greatest benefits from protein intake, but further studies are needed to make explicit recommendations”. “In combination with increased protein intake, exercise is recommended at individualized levels that are safe and tolerated”. | 44 |
| Report of the International Sarcopenia Initiative – European Working Group on Sarcopenia in Older People (EWGSOP) and International Working Group on Sarcopenia (IWGS) | 2014 | “Some nutrition interventions such as EAAs (with ∼2.5 g of leucine) and HMB may improve muscle parameters. Although our findings did not appear to support this approach, increasing protein intake to 1.2 g/kg body weight/day, either by improving diet or adding protein supplements, has been recommended for adults and older people by an expert group [44]. Evidence to recommend specific interventions is yet to be established”. | 45 |
| The Belgian Society of Gerontology and Geriatrics | 2021 | “The umbrella review provided sufficient evidence to recommend leucine supplementation for sarcopenic older people to increase muscle mass, but not for muscle strength or physical performance”. | 54 |
| The Society on Sarcopenia, Cachexia and Wasting Disorders | 2010 | “It is recommended that the total protein intake should be 1 to 1.5 g/kg/day.” “It is suggested that a leucine-enriched balanced essential amino acid mix may be added to the diet.” “A trial of balanced amino acid supplementation alone and with exercise in sarcopenia is recommended.” “Creatine may enhance the effects of exercise in sarcopenic patients.” “25(OH) vitamin D levels should be measured in all sarcopenic patients.” “Vitamin D supplementation in doses sufficient to raise levels above 100 nmol/l should be given as an adjunctive therapy.”; “Either vitamin D2 or D3 is an acceptable replacement.”; “Doses of 50,000 IU of vitamin D a week are safe.” “Short-term resistance exercise improves strength and gait speed.”; “Aerobic exercise improves quality of life years (QALY) and is cost effective.”; “Epidemiology studies suggest positive effects of physical fitness on health.”; “We recommend resistance and aerobic exercise for 20 to 30 minutes, 3 times a week.” | 161 |
| The Society on Sarcopenia, Cachexia and Wasting Disorders | 2019 | “A protein intake of 1 to 1.5 g/kg/day in conjunction with physical exercise seems reasonable for a person with sarcopenia.” “Vitamin D supplementation specifically for sarcopenia was found to have insufficient evidence, though there is evidence that persons with low vitamin D levels may improve their strength with vitamin D supplementation.” “At present, there is insufficient evidence that vitamin D, anabolic steroids, or newer pharmacological agents should be used to treat sarcopenia.” “β-hydroxy β-methylbutyrate (HMB) has been shown to improve muscle mass and to preserve muscle strength and function in older people with sarcopenia or frailty.” | 83 |
| The Asian Working Group for Sarcopenia (AWGS) | 2019 | “Available evidence suggests that exercise plus nutrition improves muscle strength and function, with variable effects on muscle mass.” “AWGS 2019 highlights the impact of sarcopenia in all health care settings and recommends individualized lifestyle intervention that may be implemented across the health care spectrum.” | 162 |
| International Conference on Sarcopenia and Frailty Research (ICSFR) | 2018 | “We recommend clinicians consider protein supplementation/a protein-rich diet for older adults with sarcopenia.” “Clinicians may also consider discussing with patients the importance of adequate calorie and protein intake.” “Nutritional (protein) intervention should be combined with a physical activity intervention.” “Insufficient evidence exists to determine whether a Vitamin D supplementation regime by itself is effective in older adults with sarcopenia.” | 160 |
| The Korean Geriatric Society and the Korean Nutrition Society | 2018 | “Based on the currently available evidence, we recommend a dietary protein intake of > 1.2 g/kg and > 20 g essential amino acids per day in healthy older adults.” “Leucine or BCAAs and β-HMB enrichment may be beneficial, although the clinical evidence is insufficient. Fast protein (for example, whey protein) may be beneficial compared to slow protein (for example, casein protein), and protein of animal origin may be better than plant-based protein in promoting muscle mass.” “Evidence indicates that the combination of timely exercises with protein intake may synergistically stimulate muscle protein synthesis, leading to improved muscle mass and strength in older people.” | 163 |
According to the Society on Sarcopenia, Cachexia and Wasting Disorders (SCWD) [83] there is a strong recommendation that individuals with sarcopenia should be enrolled into a resistance exercise program to increase both muscle mass and muscle strength. The use of a protein-rich diet (1 to 1.5 g/day) or protein supplementation received a conditional recommendation for muscle wasting treatment [44, 158, 159] and higher doses of protein (up to 2 g/day) may be appropriate in persons with severe illness or injury or when there is evidence of a pro-inflammatory/catabolic state [44, 158]. The Belgian Society of Gerontology and Geriatrics recommends leucine supplementation for sarcopenic older people to increase muscle mass, but not for muscle strength or physical performance [54]. In 2018, an international, multidisciplinary guideline development task force from the International Conference on Sarcopenia and Frailty Research (ICSFR) published Clinical Practice Guidelines for Sarcopenia. The authors recommended that clinicians should consider protein supplementation/a protein-rich diet for older adults with sarcopenia. Clinicians may also consider discussing with patients the importance of adequate calorie and protein intake. The guidelines conditionally recommended that nutritional supplementation should be combined with a physical activity intervention for older adults with sarcopenia. The experts also agreed that there was insufficient evidence to recommend a vitamin D supplementation regime for older adults with sarcopenia [160].
Based on our review on nutraceuticals in HF-associated muscle wasting, leucine (∼2.5 g daily) supplementation may be useful by improvement of the lean muscle-mass content and functional performance. Also HMB (∼3 g daily) has been shown to improve muscle mass and to preserve muscle strength and function in older patients with sarcopenia or frailty [52]. Regarding vitamin D supplementation specifically for sarcopenia, there is some evidence that people with low vitamin D levels might improve their strength with additional vitamin D intake [81]. Therefore low vitamin-D levels require replenishment, but as with protein supplementation, intervention trials of the effect of vitamin D on strength and physical performance have shown mixed results [161].
The utility of other nutraceuticals with anti-inflammatory activity such as α lipoic acid, creatine, curcumin, or beetroot in the prevention and treatment of HF-associated muscle wasting needs further studies [162, 163]. We do not recommend vitamin C and omega-3 fatty acids in this indication. An important area for further research is the potential for whole-diet interventions, which attempt to change dietary patterns rather than focusing on specific nutrients in isolation [164–177].