Introduction
Heart failure (HF) occurs when the heart is unable to pump blood effectively due to structural or functional abnormalities, leading to a set of symptoms and signs that define the condition [1]. Globally, there are approximately 26 million HF patients, posing a serious threat to life and health [2]. In the United States and Europe, about one million new cases of HF are reported annually [2]. HF represents the end stage of many cardiovascular diseases, with high mortality and prevalence rates. Overall, the prevalence of HF ranges from 6.3% to 13.3%, with a 5-year mortality rate as high as 75.4% [3]. Common causes of HF encompass a spectrum of conditions, including obesity, diabetes, myocardial ischemia, myocarditis, coronary artery disease, and arrhythmias [4, 5]. Sleep disturbances frequently serve as a common triggering factor for acute exacerbations of HF in patients during the stable phase of the condition [6]. Additionally, sleep disturbances are commonly present in patients with HF [7].
Sleep disorders manifest in various forms, including insomnia, difficulty falling asleep, trouble sleeping, and nocturnal awakening. About 10–15% of the population is affected by sleep disorders, especially common among middle-aged and elderly individuals [8]. Sleep disorders can compromise health, not only reducing quality of life but also leading to mental disorders and cardiovascular diseases such as hypertension [9, 10]. Sleep disorders contribute to endothelial dysfunction, systemic inflammation, oxidative stress, and neurohormonal imbalance, which can lead to myocardial remodeling, ventricular dysfunction, and the progression of HF [11]. Previous studies suggest a correlation between sleep disorders and HF [12]. A cohort study with over 10 years of follow-up demonstrated that insomnia is associated with the onset of heart failure, exhibiting a dose-response relationship [13]. Another study confirmed that insomnia symptoms, both individually and cumulatively, are associated with incident HF in middle-aged and older adults [14]. However, residual confounding and reverse causality present alternative explanations for the observed strong correlation between sleep disorders and HF, both of which are difficult to disentangle in traditional observational studies [15]. While studies have shown a link between sleep disturbances and HF, they are limited to establishing correlation rather than causality. Mendelian randomization (MR), by leveraging genetic properties, provides a methodological approach to facilitate causal inference [15]. A recent trend in MR studies involves utilizing genetic variants as instrumental variables to simulate a randomized controlled trial design [16, 17]. In this approach, genotypes are assigned randomly to individuals before birth, allowing for causal inferences to be drawn [16, 17]. Several pioneering MR studies have investigated the causal relationship between sleep and cardiovascular disease [18–20]. However, research on the association between sleep disturbances and HF is lacking. Therefore, further MR studies are needed to clarify the causal association between sleep disturbances and HF.
This study initially examined the association between sleep disturbances and HF through an observational study. Subsequently, it delved deeper into the causal relationship between insomnia and HF using MR studies. Our study synthesized observational research and Mendelian randomization to examine the association between insomnia and heart failure. By harnessing the extensive data available from observational studies alongside the causal inference strengths of Mendelian randomization, we sought to deliver robust and comprehensive insights into this relationship.
Material and methods
Observational study
Study population
The National Health and Nutrition Examination Survey (NHANES) is a comprehensive cross-sectional survey conducted by the National Center for Health Statistics to gather data on the noninstitutionalized U.S. civilian population. Our study used NHANES survey data collected from 2005 to 2008, which included 10,432 participants. A flowchart shows the inclusion procedure of patients in the present study (Supplementary Figure S1). All participants provided informed consent after the study protocols received approval from the Institutional Review Board (IRB) of the National Center for Health Statistics.
Definition of sleep disturbances
Sleep disturbances in this study encompassed insomnia, sleep disorders, difficulty falling asleep, trouble sleeping, and nocturnal awakening. Participants were queried regarding the frequency of experiencing trouble falling asleep and nocturnal awakening, with response options including “Always”, “Most of the time”, “Sometimes”, “Rarely”, and “Never”. In this study, “Always”, “Most of the time”, and “Sometimes” were categorized as “Yes”, while “Rarely” and “Never” were categorized as “No”. Since “Rarely” occurs only once a month, it is reclassified as “No” to simplify categorization and maintain data consistency. Participants were asked if they had ever been informed by a doctor about trouble sleeping, sleep disorders, or insomnia, with response options including “Yes” or “No”.
Other data
Potential confounders were identified a priori based on a review of the existing literature. An in-home interview was conducted to collect comprehensive information regarding the patient’s medical history and current pharmacological regimen, encompassing medications for hypertension, diabetes (both type 1 and type 2), hyperlipidemia, coronary heart disease, myocardial infarction, congestive heart failure, stroke, and angina. A physical examination included measurements of blood pressure, weight, and height, followed by calculation of body mass index (BMI) by dividing weight in kilograms by the square of height in meters.
Samples collected from the mobile examination center were stored at –20°C before being analyzed at central laboratories using standard methods for measuring high-density lipoprotein cholesterol, low-density lipoprotein (LDL) cholesterol, creatinine, and plasma glucose. LDL cholesterol and glucose levels were assessed only in a subset of survey participants who had fasted for at least 8 h prior to the survey.
Statistical analysis
Following NHANES analytic guidelines, our analyses incorporated sample weights, clustering, and stratification to ensure the findings’ generalizability to the entire U.S. population. To address NHANES’s complex sampling design, we applied suitable weights to achieve a representative sample of the U.S. national population. Weighted multivariate logistic regression analyses were performed, with insomnia, sleep disorders, difficulty falling asleep, trouble sleeping, or nocturnal awakening as the independent variable and HF as the dependent variable. The objective of these analyses was to evaluate whether sleep disturbances are independently associated with HF. Patients with incomplete primary variable data were excluded from the analysis. For categorical covariates with missing data, a distinct category was created, whereas missing continuous variables were addressed through multiple imputation techniques. The R programming language was employed to address missing data through the utilization of the Mice package, which implements multiple imputation techniques.
In our study, we used three predefined models for adjustment: Model 1: This model was unadjusted to provide a baseline estimate of the association between sleep disturbances and heart failure. Model 2: We adjusted for demographic variables, including age, sex, ethnicity/race, marital status, poverty income ratio level, and education [21]. These factors are essential as they can influence both the prevalence of sleep disturbances and heart failure risk [21]. Model 3: This model included additional clinical variables – hypertension, diabetes mellitus, coronary heart disease, heart attack, stroke, smoking, angina, alcohol consumption, systolic blood pressure, HbA1c, creatinine, LDL cholesterol, uric acid, and fasting triglyceride levels [22]. These adjustments are critical because they represent established cardiovascular risk factors and can significantly impact heart failure outcomes [22].
Analyses were performed using R version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria). A two-tailed p-value less than 0.05 was considered statistically significant.
MR study
Data sources and selection of genetic variants
We conducted a search on the IEU (https://gwas.mrcieu.ac.uk/), a comprehensive catalog containing reported associations from published GWAS studies. For exposure, we used publicly available summary statistical datasets of GWAS on sleeplessness from https://gwas.mrcieu.ac.uk/datasets/ukb-a-13/ (n = 336,965). As an outcome, we employed summary statistics from a GWAS involving 47,309 patients with HF of European descent [23]. A two-sample MR study employs genetic variants associated with gout as instrumental variables (IVs) to enhance inference. We acquired summary statistics (β coefficients and standard errors [SE]) for single-nucleotide polymorphisms (SNPs) associated with sleeplessness as IVs from GWASs.
Statistical analysis for Mendelian randomization
MR analysis necessitates genetic variants to be associated with, but not act as potential confounders of, an exposure [24]. Firstly, we evaluated the independent association of SNPs with sleeplessness. Secondly, we investigated the association between each SNP and the risk of HF. Thirdly, we integrated these findings to estimate the unconfounded causal association between sleeplessness and the risk of HF using MR analysis. We conducted a two-sample MR analysis, a method used to estimate the causal effect of an exposure (sleeplessness) on outcomes (HF), using summary statistics from GWASs [25]. This enabled us to evaluate the causal relationships between sleeplessness and HF risk, employing summary data from sleeplessness and HF GWASs with SNPs as IVs. To identify genetic loci associated with sleep disturbances, we used SNPs with p-values less than 5 × 10–8 as instrumental variables, thereby excluding weak associations. The strength of each independent variable was assessed using the F statistic (β2/SE2), with a threshold of over 10 to ensure sufficient strength.
The IVW method employs a meta-analysis approach to integrate the Wald ratio estimates of the causal effect derived from various SNPs. It yields a consistent estimate of the causal effect of the exposure on the outcome, provided that each genetic variant meets the assumptions of an IV [26]. To explore and address pleiotropy, we employed the weighted median estimator and MR-Egger regression methods. MR-Egger regression analysis examines and corrects for potential unbalanced pleiotropy by introducing a parameter for this bias, incorporating summary data estimates of causal effects from multiple individual variants. This method is robust for invalid IVs [27]. MR-Egger conducts a weighted linear regression of the gene-outcome coefficients on the gene-exposure coefficients [27]. The slope of this regression represents the estimate of the causal effect, and the intercept can be interpreted as an estimate of the average horizontal pleiotropic effect across the genetic variants [28]. The weighted median estimator yields a consistent estimate of the causal effect, even when up to 50% of the information contributing to the analysis is derived from genetic variants that are invalid IVs [29]. The weighted median estimator offers the advantage of maintaining greater precision in the estimates compared to MR-Egger analysis [29].
We evaluated heterogeneities between SNPs using Cochran’s Q statistic and funnel plots [26]. Additionally, we conducted a “leave-one-out” analysis to explore whether the causal association was influenced by a single SNP. The analyses were conducted using the TwoSampleMR package (version 0.4.25) and MRPRESSO (version 1.0) in R (version 4.1.3). The significance threshold was set at 0.05, with p < 0.05 considered statistically significant.
Results
Results of observational study
Our study comprised 10,432 individuals (Table I and Supplementary Figure S1), with 2,394 experiencing troubles sleeping and 8,038 without (Table I). The average age of the group without trouble sleeping was 46.63 years, significantly lower than that of the trouble sleeping group, which had an average age of 49.91 years (p < 0.001) (Table I). The group without trouble sleeping had a higher proportion of male individuals (50.94%) compared to the trouble sleeping group (39.28%) (Table I). The average blood glucose level in the group without trouble sleeping (5.83 mmol/l) was slightly lower than that in the trouble sleeping group (5.94 mmol/l, p = 0.36). Similarly, there were no significant differences between the groups without trouble sleeping and with trouble sleeping in terms of high-density lipoprotein cholesterol (1.38 mmol/l vs. 1.38 mmol/l, p = 0.44) and low-density lipoprotein cholesterol (2.98 mmol/l vs. 3.00 mmol/l, p = 0.74) (Table I).
Table I
Participants’ baseline information
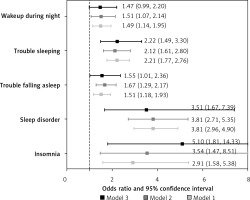
The odds ratios (OR) for HF were 2.91 (95% CI: 1.58–5.38; p = 0.001) in the group with insomnia compared to the group without insomnia (Supplementary Table SI and Figure 1). After full adjustment (model 3), the association between insomnia and HF remained significant, with an OR of 5.10 (1.81–14.33, p = 0.003) (Supplementary Table SI and Figure 1). The odds ratio (OR) for HF was 3.81 (95% CI: 2.96–4.90; p < 0.001) in the group with sleep disorders compared to the group without sleep disorders (Supplementary Table SI and Figure 1). After full adjustment (model 3), the association between sleep disorder and HF remained significant, with an OR of 3.51 (1.67–7.39; p = 0.002) (Supplementary Table SI and Figure 1). The odds ratio for HF was 1.51 (95% CI: 1.18–1.93; p = 0.002) in the group with trouble falling asleep compared to the group without trouble falling asleep (Supplementary Figure S1 and Figure 1). After full adjustment (model 3), the association between trouble falling asleep and HF remained significant, with an OR of 1.55 (1.01–2.36; p = 0.04) (Supplementary Table SI and Figure 1). The OR for HF was 2.21 (95% CI 1.77–2.76; p < 0.001) in the group with trouble sleeping compared to the group without trouble sleeping (Supplementary Table SI and Figure 1). After full adjustment (model 3), the association between trouble sleeping and HF remained significant, with an OR of 2.22 (1.49–3.30; p < 0.001) (Supplementary Table SI and Figure 1). The odds ratio (OR) for HF was 1.49 (95% CI 1.14–1.95; p = 0.005) in the group with nocturnal awakenings compared to the group without nocturnal awakenings (Supplementary Table SI and Figure 1). However, after full adjustment (model 3), the association between nocturnal awakening and HF remained significant, with an OR of 1.47 (0.99–2.20; p = 0.06) (Supplementary Table SI and Figure 1).
Results of Mendelian randomization study
25 SNPs identified in sleeplessness GWASs were chosen as IVs. These SNPs are listed in Supplementary Table SII and Figure 2. They exhibit genome-wide significant associations with sleeplessness (Supplementary Table SII). All of these SNPs showed positive associations with HF (Supplementary Table SII).
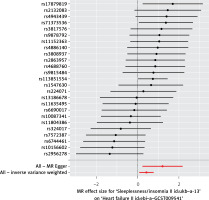
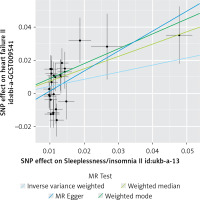
The IVW method provided evidence supporting a causal association between sleeplessness and HF (OR = 1.535, SE = 0.177, p = 0.016) (Table II and Figures 2, 3). The intercept represents the average pleiotropic effect across the genetic variants, indicating the average direct effect of a variant on the outcome. If the intercept differs significantly from zero (as determined by the MR-Egger test), it suggests evidence of directional pleiotropy. MR-Egger regression analysis indicated that directional pleiotropy was unlikely to bias the results (intercept = –0.01; p = 0.107). MR-Egger analysis demonstrated a causal association between sleeplessness and HF (OR = 3.333, SE = 0.493, p = 0.023) (Table II; Figures 2 and 3). Similarly, the weighted median approach provided evidence of a causal association between sleeplessness and HF (OR = 2.102, SE = 0.231, p = 0.001) (Table II and Figure 3). The association between sleeplessness and HF was consistent across the IVW, weighted median, weighted mode, and MR-Egger methods. The results of the MR analysis suggest a potential causal association between sleeplessness and an increased risk of HF.
Table II
MR estimates from each method of the causal effect of sleeplessness on heart failure risk
| MR method | Number of SNPs | OR | SE | Association p-value |
|---|---|---|---|---|
| MR Egger | 25 | 3.333 | 0.493 | 0.023 |
| Weighted median | 25 | 2.102 | 0.231 | 0.001 |
| Inverse variance weighted | 25 | 1.535 | 0.177 | 0.016 |
| Weighted mode | 25 | 2.458 | 0.301 | 0.006 |
Figure 3
Scatter plots of genetic associations comparing sleeplessness to the genetic associations with heart failure
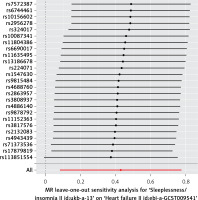
The Cochran Q-test derived P-values for MR-Egger methods indicated no heterogeneity (Q = 32.63; p = 0.087), and in the absence of a significant intercept, no directional pleiotropy was observed (Supplementary Figure S2). Results from a leave-one-out analysis demonstrated that only one single SNP (rs113851554) did not strongly violate the overall effect of sleeplessness on HF, while other single SNPs strongly violated the overall effect of sleeplessness on HF (Figure 4).
Key findings
The ORs for HF were as follows: 5.10 for insomnia, 3.51 for sleep disorder, 2.22 for trouble falling asleep, 2.22 for trouble sleeping, and 1.47 for nocturnal awakening. MR analysis using the IVW method provided evidence supporting a causal association between sleeplessness and HF, with an OR of 1.535.
Discussion
Our cross-sectional study found an association between sleep disturbances, including insomnia, sleep disorders, difficulty falling asleep, trouble sleeping, and nocturnal awakening and HF. Furthermore, MR studies have confirmed a causal relationship between insomnia and HF. Improving sleep disturbances may potentially reduce the risk of HF.
Sleep disturbances, characterized by difficulties initiating or maintaining sleep, non-restorative sleep, and early morning awakening, constitute a prevalent sleep disorder with significant implications for cardiovascular health. Previous research has indeed established a correlation between sleep disturbances and HF, which aligns with the conclusions of our study [30]. Epidemiological evidence indicates a reciprocal relationship between insomnia and HF, wherein each condition increases the risk of developing the other [31]. Sleep disturbance is a common comorbidity among individuals with HF, with prevalence rates reported to range from 30% to 70% [32, 33]. Individuals suffering from chronic insomnia are at an increased risk of developing HF, underscoring the bidirectional nature of this association [34]. Our study found a significant correlation between various sleep disorders, such as insomnia, difficulty falling asleep, trouble sleeping, nocturnal awakening, and HF. However, these findings cannot prove a causal relationship between insomnia and HF. We conducted further research to investigate the causal relationship between insomnia and HF.
Our study, employing MR analysis, confirmed the causal relationship between insomnia and HF. One potential explanation for the observed phenomena is that disruptions in sleep patterns lead to changes in the growth hormone/insulin-like growth factor-1 (GH/IGF-1) pathway [14]. In an experimental environment, rats deprived of sleep exhibited decreased serum levels of growth hormone (GH) and insulin-like growth factor-1 (IGF-1) [35]. In humans, obstructive sleep apnea syndrome was correlated with diminished secretion of GH and insulin-like IGF-1 [36]. Prior research has indicated that decreased serum concentrations of IGF-1 serve as a prognostic indicator for the likelihood of developing HF in the future [37]. Moreover, the occurrence of sleep disturbances may serve as a potential marker for depression, a known independent risk factor for HF in elderly individuals with hypertension [38].
An alternative explanation is that disruptions in sleep patterns may be associated with heightened inflammatory responses. Recent research has demonstrated elevated levels of C-reactive protein in individuals experiencing sleep deprivation [39]. Therefore, it is plausible that inflammation serves as a potential pathway through which sleep disruptions contribute to the development of HF, as elevated levels of inflammatory markers have been shown to be predictive of the onset of HF [40, 41]. The complex pathophysiological mechanisms that connect insomnia and HF are diverse [42]. Sleep disturbances have been shown to disrupt the function of the autonomic nervous system, resulting in increased sympathetic activity, decreased parasympathetic activity, and changes in heart rate variability [43]. The aforementioned physiological alterations play a role in the development of endothelial dysfunction, systemic inflammation, oxidative stress, and neurohormonal dysregulation, ultimately leading to myocardial remodeling, ventricular dysfunction, and the progression of HF [11]. These studies might elucidate the mechanisms underlying the causal association between insomnia and HF.
Our study confirms an association between sleep disorders and heart failure, with Mendelian randomization analysis establishing a causal relationship. Sleep disturbances may contribute to HF development through several pathophysiological mechanisms [31]: dysregulation of the hypothalamic-pituitary-adrenal axis, leading to insulin resistance and diabetes onset [44]; activation of the sympathetic nervous system, driven by increased cortisol and norepinephrine, leading to elevated resting heart rate, increased blood pressure, and altered heart rate variability [45]; elevated secretion of pro-inflammatory cytokines, including C-reactive protein, tumor necrosis factor-α, and interleukin-6, which promote atherosclerosis [11, 39, 46]; impaired glucose tolerance and dyslipidemia. These mechanisms may account for our findings; however, further research is required to fully elucidate these mechanisms and their interconnections.
Our research indicated a significant association between sleep disturbances and heart failure, establishing a causal relationship between these conditions. Consequently, addressing sleep disturbances could be a crucial strategy in mitigating the incidence of heart failure. Evidence-based interventions, such as cognitive behavioral therapy for insomnia (CBT-I), pharmacological treatments such as melatonin, and lifestyle modifications, may substantially reduce the risk of heart failure by targeting sleep disturbances [47–50]. When judiciously administered, pharmacological treatments can further facilitate sleep restoration while minimizing adverse effects [47]. Integrating sleep management into cardiovascular care, particularly for high-risk populations, is crucial for comprehensive disease prevention strategies [51]. The treatment of sleep disorders generally involves a comprehensive approach, with lifestyle interventions as the primary strategy, supported by medication when necessary [52].
Our findings suggest that sleep disturbances may significantly contribute to the development and progression of HF. We agree that it is crucial to focus on patients at risk of HF, particularly those with a history of acute coronary syndromes or myocardial infarctions [53]. Excessive exercise is not recommended for these patients, as the anxiety caused by their condition may exacerbate sleep disturbances. In such cases, stronger interventions, such as medications, might be effective. We also recognize the importance of addressing sleep disturbances in individuals undergoing primary prevention. Early identification and management of sleep disturbances in these patients may help reduce the risk of developing HF later in life [54]. The prevention of atherosclerotic cardiovascular disease (ASCVD) primarily focuses on lifestyle modifications (e.g., exercise, smoking cessation) and managing risk factors (hypertension, diabetes, dyslipidemia) [55]. Incorporating sleep health into the primary prevention paradigm may offer a more comprehensive approach to cardiovascular health. Overall, for patients already at risk of HF, medications may be more effective for prevention [56]. In contrast, lifestyle interventions may be more effective for primary prevention of ASCVD [57].
Personalized management and intervention for sleep disorders could be an effective strategy to reduce the burden of heart failure. Sleep assessment and management could be incorporated into the prevention and treatment protocols for heart failure and other cardiovascular diseases. It is important to emphasize that excessive sleep duration may be harmful, as prolonged sleep may increase the risk of kidney damage, which in turn could elevate the risk of heart failure [58, 59]. Healthcare providers should be encouraged to systematically evaluate and address patients’ sleep health as an integral component of cardiovascular care.
This study also has certain limitations. First, our observational study investigated the association between sleep disturbances, such as insomnia, and HF. The MR analysis primarily employed GWAS data on insomnia to examine the causal association between sleep disturbances and HF. However, these findings alone are adequate to establish both the correlation and potential causal link between sleep disturbances and HF. Second, the observational study primarily depended on self-reported data regarding sleep disturbances, potentially introducing reporting bias. Moreover, the observational study was confined to a single baseline evaluation of serum electrolytes. To mitigate these limitations, MR analysis was performed to supplement the findings of the observational study. Third, limitations of the observational study include residual confounding, time-varying confounding, unmeasured confounding, and potential selection bias. In the observational study, we adjusted for a comprehensive range of heart failure risk factors. Additionally, the MR study employed a randomization method that helped mitigate other potential confounding factors. Future experimental studies and cohort investigations are needed to enhance our understanding of the underlying relationship between sleep disturbances and HF.
In conclusion, our study identified an association between sleep disturbances, such as insomnia, sleep disorders, difficulty falling asleep, trouble sleeping, and nocturnal awakenings, and HF. Additionally, the MR study verified a causal link between insomnia and HF. Addressing sleep disturbances could potentially lower the risk of developing HF. Future longitudinal, randomized controlled, and interventional studies are necessary to validate the present findings.