Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
CLINICAL RESEARCH
JAG1-Notch signaling drives M1 macrophage polarization in osteoarthritis
1
Department of Orthopaedic Surgery, The Fourth Affiliated Hospital of Nanjing Medical University, Nanjing, China
2
Department of Traumatic Surgery, Emergency Center, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, China
These authors had equal contribution to this work
Submission date: 2025-05-09
Final revision date: 2025-09-08
Acceptance date: 2025-09-12
Online publication date: 2025-12-30
Corresponding author
Guang Li
Department of Traumatic Surgery Emergency Center Shanghai East Hospital Tongji University School of Medicine No. 150 Jimo Road Pudong New Area Shanghai, China, 200120
Department of Traumatic Surgery Emergency Center Shanghai East Hospital Tongji University School of Medicine No. 150 Jimo Road Pudong New Area Shanghai, China, 200120
Fan Sun
Department of Orthopaedic Surgery The Fourth Affiliated Hospital of Nanjing Medical University No. 298, Nanpu Road Jiangbei New District Nanjing, China, 210000
Department of Orthopaedic Surgery The Fourth Affiliated Hospital of Nanjing Medical University No. 298, Nanpu Road Jiangbei New District Nanjing, China, 210000
KEYWORDS
JAG1Notch signaling pathwayosteoarthritissynovial macrophagesM1 polarizationinflammationmacrophage polarization regulation
TOPICS
ABSTRACT
Introduction:
Osteoarthritis (OA) is characterized by chronic synovial inflammation, in which synovial macrophages (SMs) play a crucial role. The contribution of JAG1 to macrophage polarization in OA remains unclear.
Material and methods:
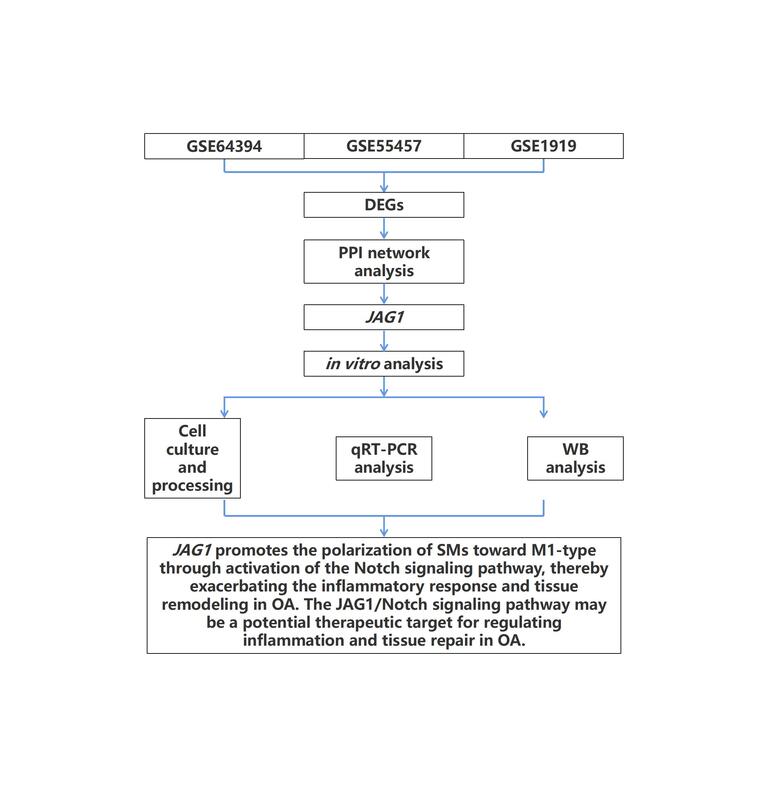
Differentially expressed genes (DEGs) were identified by analyzing the GSE64394, GSE55457, and GSE1919 datasets. An in vitro OA model was created. The effects on Notch signaling and macrophage polarization were assessed in IL-1-treated fibroblast-like synoviocytes (FLS) co-cultured with SMs. JAG1 expression was manipulated by siRNA knockdown and overexpression, and downstream effects were assessed using quantitative reverse transcription polymerase chain reaction (qRT-PCR) and Western blotting (WB).
Results:
Eleven overlapping DEGs, including JAG1, IL6, TGFB2, CX3CL1, and MMP1 were identified. Through the construction of cellular models, these genes were found to be potentially associated with inflammation, immune regulation, and extracellular matrix remodeling in OA. Additionally, the OA model was shown to induce polarization of SMs toward the M1 phenotype. In vitro experiments revealed that JAG1 was significantly upregulated in OA samples. JAG1 knockdown in the co-culture model reduced M1 markers (NOS2, PTGS2, CD64, CD86), increased M2 markers (CD206, CD163), and suppressed Notch signaling, while JAG1 overexpression reversed these effects.
Conclusions:
JAG1 promotes the polarization of SMs toward M1 in OA by activating the Notch signaling pathway, providing preliminary evidence that the JAG1/Notch axis may serve as a potential therapeutic target for regulating inflammation and tissue remodeling in OA.
Osteoarthritis (OA) is characterized by chronic synovial inflammation, in which synovial macrophages (SMs) play a crucial role. The contribution of JAG1 to macrophage polarization in OA remains unclear.
Material and methods:
Differentially expressed genes (DEGs) were identified by analyzing the GSE64394, GSE55457, and GSE1919 datasets. An in vitro OA model was created. The effects on Notch signaling and macrophage polarization were assessed in IL-1-treated fibroblast-like synoviocytes (FLS) co-cultured with SMs. JAG1 expression was manipulated by siRNA knockdown and overexpression, and downstream effects were assessed using quantitative reverse transcription polymerase chain reaction (qRT-PCR) and Western blotting (WB).
Results:
Eleven overlapping DEGs, including JAG1, IL6, TGFB2, CX3CL1, and MMP1 were identified. Through the construction of cellular models, these genes were found to be potentially associated with inflammation, immune regulation, and extracellular matrix remodeling in OA. Additionally, the OA model was shown to induce polarization of SMs toward the M1 phenotype. In vitro experiments revealed that JAG1 was significantly upregulated in OA samples. JAG1 knockdown in the co-culture model reduced M1 markers (NOS2, PTGS2, CD64, CD86), increased M2 markers (CD206, CD163), and suppressed Notch signaling, while JAG1 overexpression reversed these effects.
Conclusions:
JAG1 promotes the polarization of SMs toward M1 in OA by activating the Notch signaling pathway, providing preliminary evidence that the JAG1/Notch axis may serve as a potential therapeutic target for regulating inflammation and tissue remodeling in OA.
REFERENCES (46)
1.
Terkawi MA, Ebata T, Yokota S, et al. Low-grade inflammation in the pathogenesis of osteoarthritis: cellular and molecular mechanisms and strategies for future therapeutic intervention. Biomedicines 2022; 10: 1109.
2.
Cui L, Han Y, Dong Z. miR-106a mimics the nuclear factor-B signalling pathway by targeting DR6 in rats with osteoarthritis. Arch Med Sci 2024; 20: 302-8.
3.
Jrad AIS, Trad M, Bzeih W, El Hasbani G, Uthman I. Role of pro-inflammatory interleukins in osteoarthritis: a narrative review. Connect Tissue Res 2023; 64: 238-47.
4.
Maglaviceanu A, Wu B, Kapoor M. Fibroblast-like synoviocytes: role in synovial fibrosis associated with osteoarthritis. Wound Repair Regen 2021; 29: 642-9.
5.
Waszczykowski M, Fabiś-Strobin A, Bednarski I, Narbutt J, Fabiś J. Serum and synovial fluid concentrations of interleukin-18 and interleukin-20 in patients with osteoarthritis of the knee and their correlation with other markers of inflammation and turnover of joint cartilage. Arch Med Sci 2022; 18: 448-58.
6.
Damerau A, Rosenow E, Alkhoury D, Buttgereit F, Gaber T. Fibrotic pathways and fibroblast-like synoviocyte phenotypes in osteoarthritis. Front Immunol 2024; 15: 1385006.
7.
Panizo S, Martínez-Arias L, Alonso-Montes C, et al. Fibrosis in chronic kidney disease: pathogenesis and consequences. Int J Mol Sci 2021; 22: 408.
8.
Vázquez-Ulloa E, Lin KL, Lizano M, Sahlgren C. Reversible and bidirectional signaling of notch ligands. Crit Rev Biochem Mol Biol 2022; 57: 377-98.
9.
Karlsson C, Brantsing C, Egell S, Lindahl A. Notch1, Jagged1, and HES5 are abundantly expressed in osteoarthritis. Cells Tissues Organs 2008; 188: 287-98.
10.
Qi L, Wang M, He J, Jia B, Ren J, Zheng S. E3 ubiquitin ligase ITCH improves LPS-induced chondrocyte injury by mediating JAG1 ubiquitination in osteoarthritis. Chemicobiol Interact 2022; 360: 109921.
11.
Zhang H, Lin C, Zeng C, et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann Rheum Dis 2018; 77: 1524-34.
12.
Chen W, Liu Y, Chen J, et al. The Notch signaling pathway regulates macrophage polarization in liver diseases. Int Immunopharmacol 2021; 99: 107938.
13.
Hans CP, Sharma N, Sen S, et al. Transcriptomics analysis reveals new insights into the roles of Notch1 signaling on macrophage polarization. Sci Rep 2019; 9: 7999.
14.
Liu J, Luo R, Zhang Y, Li X. Current status and perspective on molecular targets and therapeutic intervention strategy in hepatic ischemia-reperfusion injury. Clin Mol Hepatol 2024; 30: 585-619.
15.
Condorelli AG, El Hachem M, Zambruno G, Nystrom A, Candi E, Castiglia D. Notch-ing up knowledge on molecular mechanisms of skin fibrosis: focus on the multifaceted Notch signalling pathway. J Biomed Sci 2021; 28: 36.
16.
Breikaa R. Investigating the Role of Endothelial Cell-Expressed Jag1 On Smooth Muscle Function And Vascular Homeostasis. The Ohio State University 2022.
17.
Xiao X, Wang W, Guo C, et al. Hypermethylation leads to the loss of HOXA5, resulting in JAG1 expression and NOTCH signaling contributing to kidney fibrosis. Kidney Int 2024; 106: 98-114.
18.
Li X, Li Y, Zhang W, Jet al. The IGF2BP3/Notch/Jag1 pathway: a key regulator of hepatic stellate cell ferroptosis in liver fibrosis. Clin Transl Med 2024; 14: e1793.
19.
Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 2004; 3: 3.
20.
Szklarczyk D, Kirsch R, Koutrouli M, et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res 2023; 51: D638-46.
21.
Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003; 13: 2498-504.
22.
Liu B, Xian Y, Chen X, et al. Inflammatory fibroblast-like synoviocyte-derived exosomes aggravate osteoarthritis via enhancing macrophage glycolysis. Adv Sci 2024; 11: e2307338.
23.
Dakin SG, Buckley CD, Al-Mossawi MH, et al. Persistent stromal fibroblast activation is present in chronic tendinopathy. Arthritis Res Ther 2017; 19: 16.
24.
Arunachalam K, Sasidharan SP, Arunachalam K, Sasidharan SP. Protein extraction and western blot analysis. Bioassays Exp Preclin Pharmacol 2021; 229-40.
25.
Molnar V, Matišić V, Kodvanj I, et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int J Mol Sci 2021; 22: 9208.
26.
Gonzalez-Molina J, Moyano-Galceran L, Single A, et aL. Chemotherapy as a regulator of extracellular matrix-cell communication: implications in therapy resistance. Semin Cancer Biol 2022; 86: 224-36.
27.
Zhang S, Wang P, Hu B, et al. Inhibiting heat shock protein 90 attenuates nucleus pulposus fibrosis and pathologic angiogenesis induced by macrophages via down-regulating cell migration–inducing protein. Am. J Pathol 2023; 193: 960-76.
28.
Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci 2021; 22: 6995.
29.
Motta F, Barone E, Sica A, Selmi C. Inflammaging and osteoarthritis. Clin Rev Allergy Immunol 2023; 64: 222-38.
30.
Jiang F, Zhao H, Zhang P, et al. Challenges in tendon–bone healing: emphasizing inflammatory modulation mechanisms and treatment. Front Endocrinol 2024; 15: 1485876.
31.
Zhong Y, Wei B, Wang W, et al. Single-cell RNA-sequencing identifies bone marrow-derived progenitor cells as a main source of extracellular matrix-producing cells across multiple organ-based fibrotic diseases. Int J Biol Sci 2024; 20: 5027.
32.
Ačkar L, Casjens S, Andreas A, et al. Blood-based detection of lung cancer using cysteine-rich angiogenic inducer 61 (CYR61) as a circulating protein biomarker: a pilot study. Mol Oncol 2021; 15: 2877-90.
33.
Stone AV, Loeser RF, Vanderman KS, Long DL, Clark SC, Ferguson CM. Pro-inflammatory stimulation of meniscus cells increases production of matrix metalloproteinases and additional catabolic factors involved in osteoarthritis pathogenesis. Osteoarthritis Cartilage 2014; 22: 264-74.
34.
Deroyer C, Poulet C, Paulissen G, et al. CEMIP (KIAA1199) regulates inflammation, hyperplasia and fibrosis in osteoarthritis synovial membrane. Cell Mol Life Sci 2022; 79: 260.
35.
Maldonado M, Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. BioMed Res Int 2013; 2013: 284873.
36.
Kurowska-Stolarska M, Alivernini S. Synovial tissue macrophages in joint homeostasis, rheumatoid arthritis and disease remission. Nat Rev Rheumatol 2022; 18: 384-97.
37.
Apeku E, Tantuoyir MM, Zheng R, Tanye N. Exploring the polarization of M1 and M2 macrophages in the context of skin diseases. Mol Biol Rep 2024; 51: 269.
38.
Mao J, Chen L, Cai Z, et al. Advanced biomaterials for regulating polarization of macrophages in wound healing. Adv Funct Materials 2022; 32: 2111003.
39.
Wang L, He C. Nrf2-mediated anti-inflammatory polarization of macrophages as therapeutic targets for osteoarthritis. Front Immunol 2022; 13: 967193.
40.
Fang C, Zhong R, Lu S, et al. TREM2 promotes macrophage polarization from M1 to M2 and suppresses osteoarthritis through the NF-B/CXCL3 axis. Int J Biol Sci 2024; 20: 1992.
41.
Anusewicz D, Orzechowska M, Bednarek AK. Notch signaling pathway in cancer – review with bioinformatic analysis. Cancers 2021; 13: 768.
42.
Ng C, Qin Y, Xia Y, Hu X, Zhao B. Jagged1 acts as an RBP-J target and feedback suppresses TNF-mediated inflammatory osteoclastogenesis. J Immunol 2023; 211: 1340-7.
43.
Zack SR, Meyer A, Zanotti B, et al. Notch ligands are biomarkers of anti-TNF response in RA patients. Angiogenesis 2024; 27: 273-83.
44.
Lin NY, Distler A, Beyer C, et al. Inhibition of Notch1 promotes hedgehog signalling in a HES1-dependent manner in chondrocytes and exacerbates experimental osteoarthritis. Ann Rheum Dis 2016; 75: 2037-44.
45.
Lan L, Jiang Y, Zhang W, Li T, Ying B, Zhu S. Expression of Notch signaling pathway during osteoarthritis in the temporomandibular joint. J Craniomaxillofac Surg 2017; 45: 1338-48.
46.
Pagie S, Gérard N, Charreau B. Notch signaling triggered via the ligand DLL4 impedes M2 macrophage differentiation and promotes their apoptosis. Cell Commun Signal 2018; 16: 4.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.