Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ONCOLOGY / CLINICAL RESEARCH
STXBP1 inhibits glioma progression by modulating ferroptosis and epithelial-mesenchymal transition
1
Putuo Hospital, Shanghai University of Traditional Chinese Medicine, Caoyang, Putuo District, Shanghai, China
2
Neurosurgery Department, Shanxi Province Integrated Traditional and Western Medicine Hospital, Taiyuan City, Shanxi Province, China
3
Institutes of Biomedical Sciences, Fudan University, Shanghai, China
These authors had equal contribution to this work
Submission date: 2024-11-08
Final revision date: 2025-02-15
Acceptance date: 2025-03-28
Online publication date: 2025-05-18
Corresponding author
Xuemin Li
Putuo Hospital Shanghai University of Traditional Chinese Medicine 409 Meiling North Road Caoyang, Putuo District 200063 Shanghai, China
Putuo Hospital Shanghai University of Traditional Chinese Medicine 409 Meiling North Road Caoyang, Putuo District 200063 Shanghai, China
KEYWORDS
TOPICS
ABSTRACT
Introduction:
This study investigated the role of STXBP1 in glioma, particularly its involvement in regulating ferroptosis and epithelial-mesenchymal transition (EMT), and examined its impact on glioma cell behavior.
Material and methods:
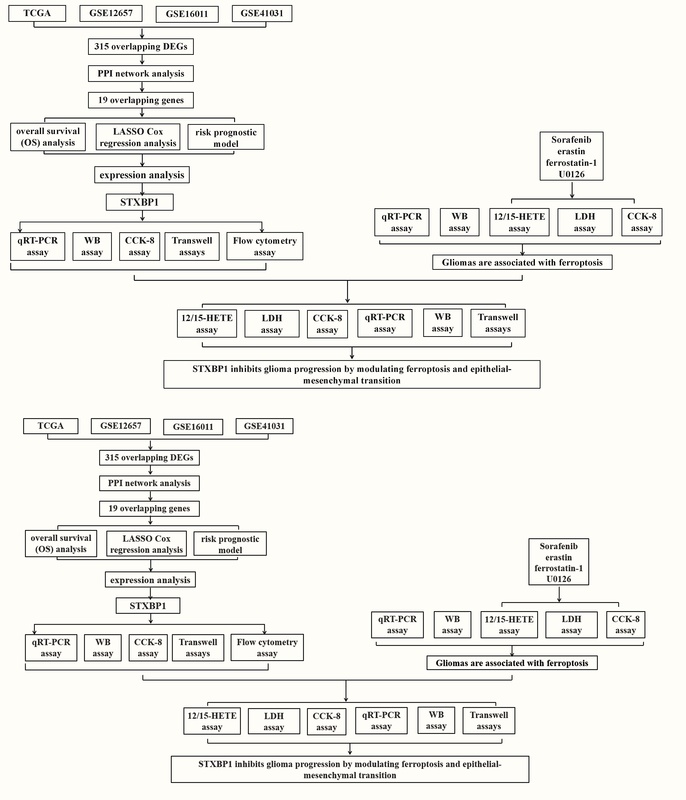
Differential gene expression analysis was performed on a glioma dataset, and protein-protein interaction (PPI) network analysis identified genes with significant prognostic value. Least absolute shrinkage and selection operator (LASSO) Cox regression analysis further narrowed the scope. Key genes were obtained through nomogram analysis, and expression verification was performed. In in vitro cell experiments, knockdown of STXBP1 was performed in glioma cell lines. The effects on cell proliferation, migration, invasion, cell cycle distribution, apoptosis, and markers of ferroptosis and EMT were assessed.
Results:
After bioinformatics analysis, STXBP1 was identified as a hub gene, and in vitro cell experiments were performed. STXBP1 knockdown in glioma cells increased proliferation, migration, and invasion, altered cell cycle distribution (reducing S phase and increasing G1 phase), and decreased apoptosis. Ferroptosis markers showed elevated GPX4 expression and reduced 12-HETE and 15-HETE levels. Ferroptosis inducers (sorafenib, erastin) heightened LDH release and reduced viability, while inhibitors (ferrostatin-1, U0126) had opposing effects. STXBP1 knockdown also reduced lipid peroxidation and mitigated the cytotoxic effects of sorafenib, indicating a role in ferroptosis regulation. Additionally, STXBP1 knockdown impacted EMT markers, decreasing N-cadherin and vimentin and increasing E-cadherin.
Conclusions:
STXBP1 functions as a tumor suppressor in glioma, regulating ferroptosis and EMT. It shows potential as a therapeutic target in glioma management.
This study investigated the role of STXBP1 in glioma, particularly its involvement in regulating ferroptosis and epithelial-mesenchymal transition (EMT), and examined its impact on glioma cell behavior.
Material and methods:
Differential gene expression analysis was performed on a glioma dataset, and protein-protein interaction (PPI) network analysis identified genes with significant prognostic value. Least absolute shrinkage and selection operator (LASSO) Cox regression analysis further narrowed the scope. Key genes were obtained through nomogram analysis, and expression verification was performed. In in vitro cell experiments, knockdown of STXBP1 was performed in glioma cell lines. The effects on cell proliferation, migration, invasion, cell cycle distribution, apoptosis, and markers of ferroptosis and EMT were assessed.
Results:
After bioinformatics analysis, STXBP1 was identified as a hub gene, and in vitro cell experiments were performed. STXBP1 knockdown in glioma cells increased proliferation, migration, and invasion, altered cell cycle distribution (reducing S phase and increasing G1 phase), and decreased apoptosis. Ferroptosis markers showed elevated GPX4 expression and reduced 12-HETE and 15-HETE levels. Ferroptosis inducers (sorafenib, erastin) heightened LDH release and reduced viability, while inhibitors (ferrostatin-1, U0126) had opposing effects. STXBP1 knockdown also reduced lipid peroxidation and mitigated the cytotoxic effects of sorafenib, indicating a role in ferroptosis regulation. Additionally, STXBP1 knockdown impacted EMT markers, decreasing N-cadherin and vimentin and increasing E-cadherin.
Conclusions:
STXBP1 functions as a tumor suppressor in glioma, regulating ferroptosis and EMT. It shows potential as a therapeutic target in glioma management.
REFERENCES (47)
1.
Smith HL, Wadhwani N, Horbinski C. Major features of the 2021 WHO classification of CNS tumors. Neurotherapeutics 2022; 19: 1691-704.
2.
Pienkowski T, Kowalczyk T, Kretowski A, Ciborowski M. A review of gliomas-related proteins. Characteristics of potential biomarkers. Am J Cancer Res 2021; 11: 3425.
3.
Nandihal P, Shetty V, Guha T, Pareek PK, editors. Glioma Detection using Improved Artificial Neural Network in MRI Images. 2022 IEEE 2nd Mysore Sub Section International Conference (MysuruCon); 2022: IEEE.
4.
Yoda RA, Cimino PJ, editors. Classification and Grading of Central Nervous System Tumors According to the World Health Organization 5th Edition. Seminars in Neurology; 2023: Thieme Medical Publishers, Inc.
6.
Sharma A, Graber JJ. Overview of prognostic factors in adult gliomas. Ann Palliat Med 2021; 10: 863-74.
7.
Jiang T, Nam DH, Ram Z, et al. Clinical practice guidelines for the management of adult diffuse gliomas. Cancer Letters 2021; 499: 60-72.
8.
Śledzińska P, Bebyn MG, Furtak J, Kowalewski J, Lewandowska MA. Prognostic and predictive biomarkers in gliomas. Int J Mol Sci 2021; 22: 10373.
9.
Freibauer A, Wohlleben M, Boelman C. STXBP1-related disorders: clinical presentation, molecular function, treatment, and future directions. Genes 2023; 14: 2179.
10.
Luo Y, Tian G, Fang X, Bai S, Yuan G, Pan Y. Ferroptosis and its potential role in glioma: from molecular mechanisms to therapeutic opportunities. Antioxidants 2022; 11: 2123.
11.
Zhu M, Wu P, Li Y, Zhang L, Zong Y, Wan M. Synergistic therapy for orthotopic gliomas via biomimetic nanosonosensitizer-mediated sonodynamic therapy and ferroptosis. Biomaterials Sci 2022; 10: 3911-23.
12.
Zhou L, Jiang Z, Shi Z, et al. New autophagy-ferroptosis gene signature predicts survival in glioma. Front Cell Develop Biol 2021; 9: 739097.
13.
Zheng Y, Ji Q, Xie L, et al. Ferroptosis-related gene signature as a prognostic marker for lower-grade gliomas. J Cell Mol Med 2021; 25: 3080-90.
14.
Zhou Y, Qian W, Li X, Wei W. NF-B inhibitor myrislignan induces ferroptosis of glioblastoma cells via regulating epithelial-mesenchymal transformation in a slug-dependent manner. Oxid Med Cell Longev 2023; 2023: 7098313.
15.
Abramov D, Guiberson NGL, Burré J. STXBP1 encephalopathies: clinical spectrum, disease mechanisms, and therapeutic strategies. J Neurochem 2021; 157: 165-78.
16.
Zhang Y, Wang R, Liu Z, et al. Distinct genetic patterns of shared and unique genes across four neurodevelopmental disorders. Am J Med Genet B Neuropsychiatr Genet 2021; 186: 3-15.
17.
Zhou P, He N, Zhang JW, et al. Novel mutations and phenotypes of epilepsy-associated genes in epileptic encephalopathies. Genes Brain Behav 2018; 17: e12456.
18.
Stamberger H, Nikanorova M, Willemsen MH, et al. STXBP1 encephalopathy: a neurodevelopmental disorder including epilepsy. Neurology 2016; 86: 954-62.
19.
Aslan M, Hsu EC, Liu S, Stoyanova T. Quantifying the invasion and migration ability of cancer cells with a 3D Matrigel drop invasion assay. Biol Methods Protoc 2021; 6: bpab014.
20.
Markouli M, Papachristou A, Politis A, Boviatsis E, Piperi C. Emerging role of the Slit/Roundabout (Robo) signaling pathway in glioma pathogenesis and potential therapeutic options. Biomolecules 2024; 14: 1231.
21.
Wang LM, Englander ZK, Miller ML, Bruce JN. Malignant glioma. Human Brain and Spinal Cord Tumors: From Bench to Bedside. Volume 2: The Path to Bedside Management. Springer 2023; 1-30.
22.
Liu A, Jiang B, Song C, et al. Isoliquiritigenin inhibits circ0030018 to suppress glioma tumorigenesis via the miR-1236/HER2 signaling pathway. MedComm 2023; 4: e282.
23.
Yan Y, Luo A, Liu S, et al. METTL3-mediated LINC00475 alternative splicing promotes glioma progression by inducing mitochondrial fission. Research 2024; 7: 0324.
24.
Zhen W, Shan X, Cui X, et al. Exploration and validation of m7G-related genes as signatures in the immune microenvironment and prognostic indicators in low-grade glioma. Am J Transl Res 2023; 15: 3882.
25.
Kajana X, Spinelli S, Garbarino A, et al. Identification of central nervous system oncologic disease biomarkers in evs from cerebrospinal fluid (CSF) of pediatric patients: a pilot neuro-proteomic study. Biomolecules 2023; 13: 1730.
26.
Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol 2020; 21: 678-95.
27.
Obeng E. Apoptosis (programmed cell death) and its signals - a review. Brazil J Biol 2020; 81: 1133-43.
28.
Yang Y, Liu Z, Lu Y, et al. Rab3a attenuates spinal cord injury by mediating vesicle release. Brain Res Bull 2024; 208: 110884.
29.
Lv Y, Zhang C, Jian H, et al. Regulating DNA methylation could reduce neuronal ischemia response and apoptosis after ischemia-reperfusion injury. Gene 2022; 837: 146689.
30.
Bhawe K, Felty Q, Yoo C, et al. Nuclear respiratory factor 1 (NRF1) transcriptional activity-driven gene signature association with severity of astrocytoma and poor prognosis of glioblastoma. Mol Neurobiol 2020; 57: 3827-45.
31.
Wang L, Fang X, Ling B, et al. Research progress on ferroptosis in the pathogenesis and treatment of neurodegenerative diseases. Front Cell Neurosci 2024; 18: 1359453.
32.
Zuo Z, Liu W, Zeng Y, et al. Multiparametric magnetic resonance imaging-derived deep learning network to determine ferroptosis-related gene signatures in gliomas. Front Neurosci 2022; 16: 1082867.
33.
Zhuo S, Chen Z, Yang Y, Zhang J, Tang J, Yang K. Clinical and biological significances of a ferroptosis-related gene signature in glioma. Front Oncol 2020; 10: 590861.
34.
Liu J, Kang R, Tang D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J 2022; 289: 7038-50.
35.
Kuang F, Liu J, Tang D, Kang R. Oxidative damage and antioxidant defense in ferroptosis. Front Cell Develop Biol 2020; 8: 586578.
36.
Li B, Chen X, Qiu W, et al. Synchronous disintegration of ferroptosis defense axis via engineered exosome-conjugated magnetic nanoparticles for glioblastoma therapy. Adv Sci 2022; 9: e2105451.
37.
Zhao Y, Fei Y, Zhao Y, et al. Biomineralization-tuned nanounits reprogram the signal transducer and activator of transcription 3 signaling for ferroptosis-immunotherapy in cancer stem cells. ACS Nano 2024; 18: 21268-87.
38.
Zhao Y, Wang Q, Zhu J, et al. Identification of KW-2449 as a dual inhibitor of ferroptosis and necroptosis reveals that autophagy is a targetable pathway for necroptosis inhibitors to prevent ferroptosis. Cell Death Dis 2024; 15: 764.
39.
Han L, Peng K, Qiu LY, et al. Hitchhiking on controlled-release drug delivery systems: opportunities and challenges for cancer vaccines. Front Pharmacol 2021; 12: 679602.
40.
Chen BZ, He YT, Zhao ZQ, et al. Strategies to develop polymeric microneedles for controlled drug release. Adv Drug Deliv Rev 2023: 115109.
41.
Navas T, Kinders RJ, Lawrence SM, et al. Clinical evolution of epithelial–mesenchymal transition in human carcinomas. Cancer Res 2020; 80: 304-18.
42.
Santarosa M, Maestro R. The autophagic route of e-cadherin and cell adhesion molecules in cancer progression. Cancers 2021; 13: 6328.
43.
Choi S, Yu J, Kim W, Park KS. N-cadherin mediates the migration of bone marrow-derived mesenchymal stem cells toward breast tumor cells. Theranostics 2021; 11: 6786.
44.
Usman S, Waseem NH, Nguyen TKN, et al. Vimentin is at the heart of epithelial mesenchymal transition (EMT) mediated metastasis. Cancers 2021; 13: 4985.
45.
Zou S, Zhang D, Xu Z, Wen X, Zhang Y. JMJD3 promotes the epithelial mesenchymal transition and migration of glioma cells via the CXCL12/CXCR4 axis. Oncol Letters 2019; 18: 5930-40.
46.
Zou M, Duan Y, Wang P, et al. DYT-40, a novel synthetic 2-styryl-5-nitroimidazole derivative, blocks malignant glioblastoma growth and invasion by inhibiting AEG-1 and NF-B signaling pathways. Sci Rep 2016; 6: 27331.
47.
Zou M, Zhu W, Wang L, et al. AEG-1/MTDH-activated autophagy enhances human malignant glioma susceptibility to TGF-1-triggered epithelial-mesenchymal transition. Oncotarget 2016; 7: 13122.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.