Introduction
Lipid disorders, mainly hypercholesterolemia, are in terms of prevalence, the third – after elevated blood pressure and dietary risks- most common cardiovascular risk factor in the world [1], while in Poland and in most European countries they rank first (current data indicate that 70% of Poles – 21 million – suffer from hypercholesterolemia) [2–4]. Elevated serum low-density lipoprotein cholesterol (LDL-C) concentration has persisted as a leading modifiable risk factor and is one of the most closely linked markers of atherosclerotic cardiovascular disease (ASCVD) [5]. In 2021, 3.81 million (95% CI: 2.17–5.42 million) cardiovascular (CV) deaths and 3.81 million (95% CI: 2.17–5.42 million) deaths overall were attributed to elevated LDL-C serum concentrations, and these data may represent underestimates [6]. Between 1990 and 2019, the prevalence of CVD increased significantly from 271 million to 523 million. At the same time, the number of CVD-related deaths also increased from 12.1 million to 18.6 million [1]. In 2020, approximately 19.1 million deaths were attributed to CVD globally [4]. Almost 2/3 (67.3%) of all CVDs are attributable to ASCVD, mainly CAD, ischemic stroke, and peripheral arterial disease, and, therefore in many cases a significant percentage of this mortality might be preventable [1]. In this context, lipid disorders and ASCVD are the leading cause of premature death worldwide [7].
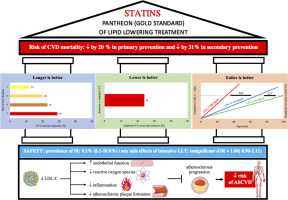
Lipid-lowering therapy is the most efficacious approach to primary and secondary prevention of ASCVD [2, 3]. Lipid-lowering therapy in primary prevention is associated with a reduction in the risk of death from any cause by 11%, CV death by 20%, acute coronary syndrome by 38%, stroke by 17%, unstable CAD by 25% and major CV events by 26% [8]. In secondary prevention, lipid-lowering treatment leads to a reduction in the risk of death from any cause by 22%, death from CV causes by 31%, acute coronary syndrome by 38%, the need for coronary revascularization by 44% and cerebrovascular events by 25% [9]. At the same time statin therapy and lipid lowering combination therapy (with an adherence of 97–100% for PCSK9 inhibitors and inclisiran) are among the most well tolerated medications used to manage CVD. Remarkably, 93-95% of patients can be treated without safety concerns [10–14].
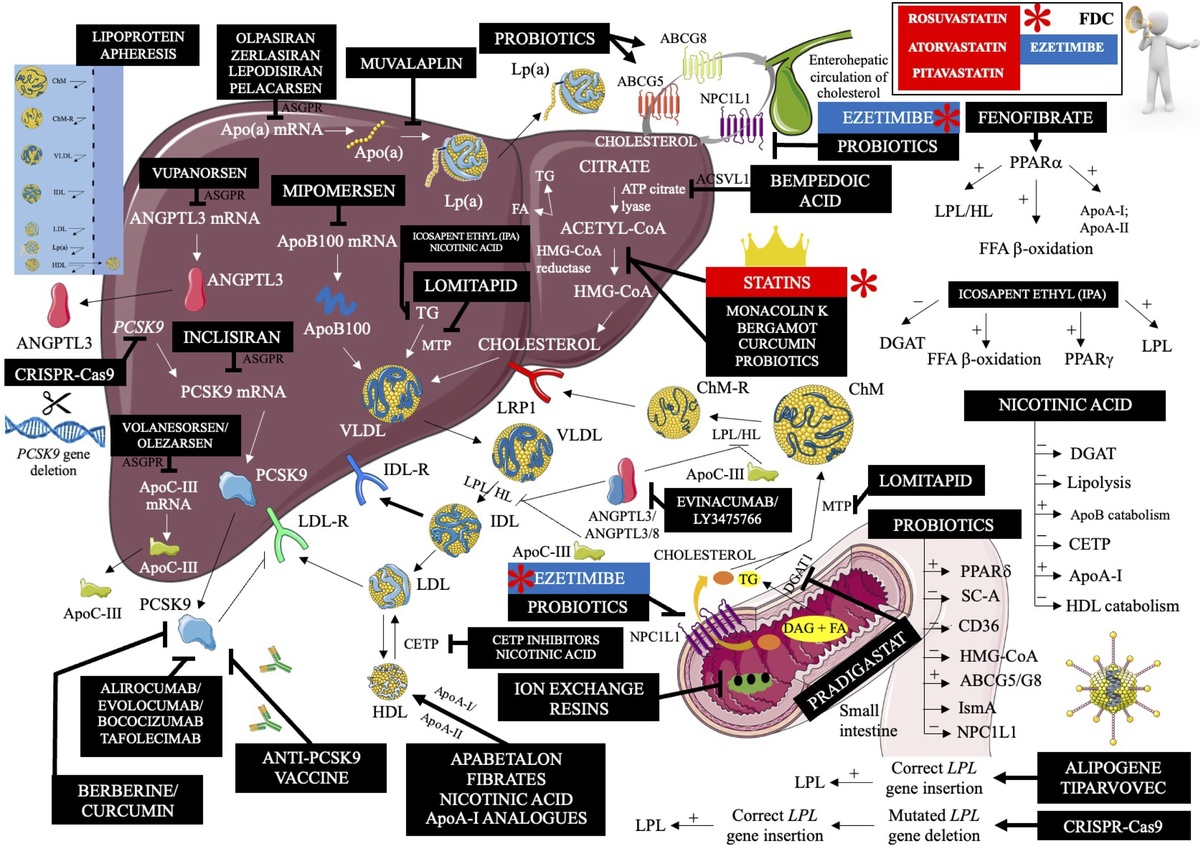
Patients accrue the greatest benefits from lipid-lowering treatment in accordance with three key principles. First, the earlier we start treatment of lipid disorders, the better (“the earlier the better”). The study by Domanski et al, which included 4958 participants aged 18–30 years followed for 16 years, found that the risk of ASCVD was higher in those who were exposed to elevated serum LDL-C concentrations at a younger age compared to people exposed to elevated LDL-C in old age, which emphasizes the importance of optimal control of serum LDL-C concentration from an early age [15]. This is also confirmed by the results of a study by Zhang et al, including 18,288 participants, which concluded that cumulative LDL-C exposure during young adulthood and middle age were associated with the risk of incident ASCVD, independent of midlife LDL-C serum concentrations [16]. Second, the treatment of lipid disorders should be intensive, according to the principle “the lower the better” with a number of studies, trials and meta-analyses confirming this relationship [17–20]. The greater benefits of more intensive lipid-lowering treatment are also confirmed by a meta-analysis of 18 randomized clinical trials (RCTs) which shows that more intensive reduction of LDL-C was associated with an additional reduction in the risk of CVD events by 24% (RR = 0.76; 95% CI: 0.68–0.85) and the risk of death from any cause by 10% (0.90; 0.83–0.97) [21]. Third, lipid-lowering treatment should be ongoing for a lifetime, according to the principle “the longer the better”. Each 1% reduction in serum LDL-C is associated with an approximately 1% reduction in cardiovascular risk. After 5 years, the risk decreases by approximately 20–25%, and after 40 years by even 50–55% for each mmol/l of LDL-C reduction [20]. A meta-analysis of 21 studies also showed that the longer lipid-lowering treatment lasts, the greater the cardiovascular benefits. Each mmol/l reduction in serum LDL-C is associated with a 12% (95% CI: 8–16%) reduction in the risk of major CV events at year 1, 20% (16–24%) in year 3, 23% (18–27%) in year 5, and 29% (14–42%) in year 7 of lipid-lowering treatment [22]. Figure 1 summarizes the most important information regarding lipid-lowering treatment.
Figure 1
Basics of lipid-lowering treatment – method of management, impact on prognosis, safety, and biological mechanisms. Based on information from [2, 3, 8, 11–18]
CVD – cardiovascular disease, LLT – lipid-lowering treatment, CV – cardiovascular, ASCVD – atherosclerotic cardiovascular disease, SI – statin intolerance, OR – odds ratio, LDL-C – low density lipoprotein cholesterol.
It should be emphasized that, in addition to the control of LDL-C concentration in serum, it is also very important to assess the levels of other lipid parameters, such as: triglycerides, lipoprotein (a) [Lp(a)], non-HDL-C cholesterol and apolipoprotein B (apoB), in order to reduce residual CVD risk [3, 23].
Despite the data demonstrating that lipid-lowering treatment prolongs the life of most patients in the primary and secondary prevention of ASCVD, risk-stratified, guideline directed control of LDL-C is not the case in 75% individuals [24–27]. Only 1/3 patients in Europe, and 1/4 in Poland and Central and Eastern Europe, reach their LDL-C targets [24, 25]. The therapeutic goal for patients with very high cardiovascular risk (i.e, < 55 mg/dl/< 1.4 mmol/l) is achieved only in 18% of the European population, 17% in Poland and only 13% in the countries of Central and Eastern Europe [24, 25]. In turn, the LDL-C target in the group of patients with extreme cardiovascular risk (i.e, < 40 mg/dl; < 1 mmol/l) is achieved by less than 10% of patients [24, 25]. When trying to find the most common reasons for this low rate of therapeutic success, two issues seem to be the most important: use of moderately intensive statin therapy in over 50% of patients and severe underutilization of lipid lowering combination therapy (< 10%) [24–29]. The more recent SANTORINI study, which included 9044 patients with high or very high cardiovascular risk from 14 western European countries, showed only a slight improvement, as only 20.1% achieved the LDL-C target (24% of high-risk patients and 18.6% of very high-risk patients) [28]. Moreover, it was found that 21.8% of patients did not receive any lipid-lowering treatment (23.5% and 21.1%). In this study, 54.3% of patients (58.4% and 52.5%) used statin monotherapy and combination therapy was used in 24% of patients (18.1% and 26.4%). The most commonly used combination was a statin with ezetimibe [28].
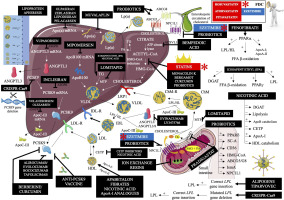
We have many effective lipid lowering therapies (LLTs) at our disposal. This offers the possibility of therapy personalization after considering the presence of concomitant risk factors and specific forms of dyslipidemia/mixed dyslipidemia. New medications for the management of dyslipidemia will continue to be developed, and available lipid-lowering treatment are so effective that most patients should be able to achieve their therapeutic goals. We believe inadequately treated dyslipidemias should be a rare event. we have no doubt that 2023 should be established as the year of new and prospective lipid lowering therapies. Figure 2 shows the mechanism of action of lipid-lowering drugs, therapeutic strategies, and nutraceuticals available or under investigation.
Figure 2
Mechanism of action of lipid-lowering drugs (available and in clinical trials), major nutraceuticals and other therapeutic strategies
Apo(a) – apolipoprotein (a), mRNA – messenger ribonucleic acid, ANGPTL3 – angiopoietin like protein 3, CRISPR-Cas9 – clustered regularly interspaced short palindromic repeats – caspase 9, ASGPR –asialoglycoprotein receptor, ApoC-III – apolipoprotein CIII, ApoB100 – apolipoprotein B100, PCSK9 – proprotein convertase subtilisin/kexin 9, VLDL – very low-density lipoprotein, LDL-R – low-density lipoprotein receptor, Lp(a) – lipoprotein (a), TG – triglycerides, MTP – microsomal triglyceride transfer protein, IDL-R – intermediate-density lipoprotein receptor (VLDL remnants), LDL – low-density lipoprotein, CETP – cholesterol ester transfer protein, HDL – high-density lipoprotein, ApoA-I – apolipoprotein A-I, LRP1 – low density lipoprotein receptor-related protein 1, LPL – lipoprotein lipase, HL – hepatic lipase, IDL – intermediate-density lipoprotein (VLDL remnants), ABCG5/8 – ATP binding cassette subfamily G member 5/8, acetyl-CoA – acetyl coenzyme A, FDC – fixed dose combination, HMG-CoA – β-hydroxy β-methylglutaryl-CoA, FA – fatty acid, NPC1L1 – Niemann-Pick C1-Like 1, ACSVL1 – very long-chain acyl-CoA synthetase, ChMR – chylomicron remnants, ChM – chylomicron, PPARa – peroxisome proliferator-activated receptor α, FFA – free fatty acid, ApoA-II – apolipoprotein A-II, DGAT – diglyceride acyltransferase, PPARg – peroxisome proliferator-activated receptor g, PPARd – peroxisome proliferator-activated receptor d, SC-A – scavenger receptor A, CD36 – cluster of differentiation 36, IsmA – intestinal sterol metabolism A. Modified and updated with permission based on: Surma S, Burchardt P, Banach M, Leki hipolipemizujące. In: Kardiologia - Podręcznik Polskiego Towarzystwa Kardiologicznego. Wydanie II, Via Medica 2023, Rycina 2.1.13.1, page 133.
Upfront lipid lowering combination therapy of high intensity statin (HIS) therapy with ezetimibe
In patients with very high and extremely high CV risk, LLT should be started with combination therapy (preferably a fixed dose combination (FDC) of high intensity statin (HIS) with ezetimibe – Figure 2) [29, 30]. A meta-analysis of 14 studies showed that the use of HIS with ezetimibe versus HIS monotherapy allowed for an additional reduction in serum LDL-C concentration by 14% and more patients attaining their LDL-C goal [31]. The RAndomized Comparison of Efficacy and Safety of Lipid-lowerING With Statin Monotherapy Versus Statin Ezetimibe Combination for High-risk Cardiovascular Diseases (RACING) trial, which included 3780 patients with ASCVD during a 3-year follow-up, showed that the use of combination therapy with a moderate intensity statin (MIS; rosuvastatin 10 mg) and ezetimibe was associated with a higher percentage of achieving serum LDL-C concentrations < 70 mg/dl (1.8 mmol/l) compared to rosuvastatin 20 mg in monotherapy (72% vs. 58%, p < 0.0001) [32]. Moreover, combination therapy was associated with a significantly lower risk of therapy discontinuation or drug dose reduction (4.8% vs. 8.2%, p < 0.0001), while maintaining the same cardiovascular benefits (with the reduction trend – the absolute difference between group was –0.78%) [32]. Older people have a higher risk of intolerance, nonadherence, and discontinuation with HIS therapy [33]. In a subgroup analysis of the RACING study including patients with ASCVD aged ≥ 75 years, it was found that the use of combination therapy with a MIS with ezetimibe for 3 years was associated with a lower risk of intolerance compared to HIS monotherapy (2.3% vs. 7.2 %, p = 0.01), while maintaining the same cardiovascular benefits (HR = 0.87; 95% CI: 0.54–1.42) [34]. Another group at greater risk of intolerance to lipid-lowering treatment are diabetic patients [13, 35]. In a subgroup analysis of the RACING study with 1398 diabetic patients, it was found that the use of lipid lowering combination therapy was associated with a lower incidence of intolerance (5.2% vs. 8.7%, p = 0.014), and higher percentage of goal attainment < 70 mg/dl (1.8 mmol/) (79.9% vs. 66.8%, p < 0.0001) [36]. In this concept, it is worth mentioning the study that used the inclusion criteria of the RACING trial that investigated the role of lipid lowering combination therapy in patients who underwent percutaneous coronary intervention (PCI) [37]. The study included 72,050 patients and showed that the use of MIS combination therapy with ezetimibe vs. HIS monotherapy was associated with a lower risk of the primary endpoint of CVD death, myocardial infarction (MI), coronary artery revascularization, hospitalization for heart failure (HF) treatment, or nonfatal stroke (HR = 0.75; 95% CI: 0.70–0.79), lower risk of discontinuation of therapy (0.85; 95% CI: 0.78–0.94), and lower risk of new onset diabetes (NOD) (0.80; 95% CI: 0.72–0.88) [37]. In a recent study by Lewek et al, including 38,023 patients with a history of an ACS from the Polish Registry of Acute Coronary Syndromes (PL-ACS), the impact of statin combination therapy with ezetimibe vs. statin monotherapy (mostly on HIS) on long-term prognosis was analyzed [38]. After 3-year follow-up the authors showed that upfront combination therapy was associated with a significantly greater reduction in the risk of all-cause mortality compared to statin monotherapy (OR = 0.526; 95% CI: 0.378–0.733) with absolute risk reduction (ARR) of 4.7% and number needed to treat (NNT) = 21. It is worth emphasizing that the significant reduction in mortality appeared after only 52 days of the lipid lowering combination therapy (ARR 1%, p = 0.032) [38].
In an RCT by Mehta et al. (EPIC-STEMI) with ST segment elevating myocardial infarction (STEMI) patients undergoing percutaneous coronary intervention (PCI), the effect of HIS with alirocumab vs. HIS on serum LDL-C concentrations was assessed. It was shown that early use of alirocumab allowed for an additional reduction in serum LDL-C concentration by 22.3% after 45 days after PCI. This resulted in a higher percentage of patients achieving the LDL-C target in the HIS + alirocumab group (92.1% vs. 56.7%, p < 0.001). Adding alirocumab to HIS after PCI also allowed for a faster lipid-lowering effect (during the first 24 h – 0.01 mmol/l/h, p = 0.03) compared to HIS monotherapy [39]. In the Virginia Commonwealth University Alirocumab Response Trial (VCU-AlirocRT), the addition of alirocumab to HIS was assessed in patients with non-ST segment elevating myocardial infarction (NSTEMI) within 24 h of diagnosis [40]. After 72 h, the serum LDL-C concentration was significantly lower in the group that received alirocumab (73 mg/dl vs. 94 mg/dl, p = 0.02). It was shown that early addition of alirocumab allowed for an additional reduction in serum LDL-C concentration by 62 mg/dl 14 days after administration (28 mg/dl vs. 90 mg/dl, p < 0.001) [40].
The Evolocumab in Acute Coronary Syndrome (EVACS) trial, including 57 patients with type 1 NSTEMI, evaluated the effect of administering evolocumab as an add-on to HIS within 24 h of diagnosis [41]. It was shown that LDL-C was significantly reduced just 24 h after evolocumab administration (91.5 mg/dl vs. 70.4 mg/dl, p < 0.01). Moreover, 3 days after the administration of evolocumab, some patients were already at therapeutic goal of < 70 mg/dL (LDL-C 49.2 mg/dl vs. 76.1 mg/dl, p = 0.02). After 30 days, the LDL-C reduction was significantly lower in patients who received evolocumab in addition to HIS (35.9 mg/dl vs. 64.5 mg/dl, p < 0.01) [41]. Similar results were provided by the EVOlocumab for Early Reduction of LDL-cholesterol Levels in Patients with Acute Coronary Syndromes (EVOPACS) trial, including 308 patients with ACS. It was shown that the use of HIS with evolocumab, compared to HIS monotherapy, led to a reduction in serum LDL-C concentration after 8 weeks by 40.7%, which was related to the fact that 95.7% of patients were in at the therapeutic target (vs. 37.6% in HIS monotherapy) [42]. Recent evidence also demonstrated the benefits of early PCSK9 inhibitors in lipid reduction, plaque stabilization, and short-term or long-term cardiovascular event prevention [43].
All the aforementioned results are reflected in the 2023 ESC guidelines on the management of patients with ACS [44], in which for the first time the recommendation of the upfront lipid lowering combination therapy was indicated with a IIb class of recommendations. We might be arguing that the level should be IIa at least, but nevertheless it is a critically important step forward to recommend effective LLT in very high risk and extremely high-risk patients with ACS [44].
Despite the proven effectiveness of HIS combination therapy with ezetimibe and PCSK9 inhibitors, 24% of physicians deescalate the statin dose by adding ezetimibe or PCSK9i [45, 46], thereby reducing the expected percentage of patients reaching their risk-stratified goal for LDL-C. Thus, it is critically important to emphasize that while adding ezetimibe or PCSK9i to HIS, the statin dose not be reduced unless the patient is experiencing statin-related side effects [47–49].
To sum up, in 2023 we have no doubt that immediate treatment with two drugs or even three drugs (if it is allowed by the reimbursement criteria) is a treatment that results in significantly more patients being at or below LDL-C goal with significantly fewer side effects and therapy discontinuation. As long as effort is made to prevent treatment discontinuation and de-escalation, the management of dyslipidemia with multiple drug regimens will result in substantial improvement in CVD event rate reductions compared to drug monotherapy (Figures 2–4).
Figure 3
Lipid-lowering treatment algorithm in patients with high or very high cardiovascular risk now and the possible prospective management, including drugs being under investigations
1Pitavastatin is preferable in patients with risk factors of diabetes or with diabetes; 2IPE is still not available in most of the countries in Europe. *In patients with severe hypertriglyceridemia and/or familial chylomicronaemia syndrome (FCS). FDC – fixed dose combination, LLT – lipid lowering treatment, TGs – triglycerides, PCSK9m – proprotein convertase subtilisin/kexin 9 modulator.
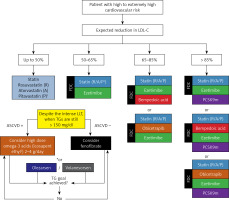
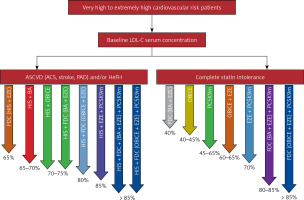
Figure 4
Lipid-lowering combination therapy in very high cardiovascular risk patients now, and the possible prospective combinations, including drugs being under investigations (*for some of the presented combinations the suggested reduction is assumption based on the available data)
ASCVD – atherosclerotic cardiovascular disease, ACS – acute coronary syndrome, PAD – peripheral artery disease, HeFH - heterozygous familial hypercholesterolemia, FDC – fixed dose combination, HIS – high intensity statin, EZE – ezetimibe, BA – bempedoic acid, OBICE – obicetrapib, PCSK9m – proprotein convertase subtilisin/kexin 9 modulator.
Bempedoic acid
Bempedoic acid, first in-class adenosine 5’-diphosphate (ATP) citrate lyase inhibitor (Figure 2), reduces the serum concentration of total cholesterol by an average of 15%, non-HDL-C by 18%, LDL-C by 23% (19–27%; approx. 40% in combination with ezetimibe), apoB by 15%, HDL-C by 6%, triglycerides by 1.5%, and apoA-I by 1.83%. The unique effect of this drug is also the reduction of high sensitivity C-reactive protein (hsCRP) by an average of 27% (> 40% in participants with high baseline hsCRP concentrations) [50, 51]. Recent analyses suggest that a stronger lipid-lowering effect of bempedoic acid is observed in women [52], and the anti-inflammatory effect of bempedoic acid is independent of background statin therapy [53]. The effect of bempedoic acid on cardiovascular risk was first assessed in the CLEAR Outcomes randomized trial, which included 13,970 patients at high cardiovascular risk with statin intolerance. Bempedoic acid 180 mg/day over a 40.6-month follow-up period was associated with a 13% reduction in the risk of the primary 4-component endpoint. In addition, fatal or non-fatal myocardial infarction (HR = 0.77; 95% CI: 0.66–0.91) and the need for coronary revascularization (0.81; 0.72–0.92) were reduced significantly as pre-specified independent endpoints [54]. This effect was even pronounced in relation to the total (not the first) number of events for primary (0.80; 0.72–0.89) and secondary endpoints (0.83; 0.73–0.93), as well as for risk reduction of 2nd (0.74; 0.63–0.87), 3rd (0.69; 0.51–0.93) and ≥ 4th CVD events (0.52; 0.31–0.88) [55]. A meta-analysis of phase-3 studies showed a beneficial effect of bempedoic acid on glycemic parameters and even significant reduction in the risk of de novo diabetes (OR = 0.59; 95% CI: 0.39–0.90) [56]. The pooled analysis also indicated that in patients with existing diabetes and lipid disorders, bempedoic acid improved glycemic control (reduced HbA1c percentage) with more patients with HbA1c < 6.5% (p = 0.03) [57]. The CLEAR Outcomes study also confirmed the observed neutral effect on glycemic parameters as well as the lack of NOD risk increase (with total 3% absolute NOD risk reduction) [54] and the most recent subgroup analysis confirmed this indicating the largest benefit of CVD events reduction (MACE-4 endpoint) in patients with diabetes (HR = 0.83; 0.72–0.85; ARR = 2.4%) in comparison to those without (0.84; 0.63–1.10; ARR = 1.9%) and with pre-diabetes (0.94; 0.81–1.09; ARR = 0.6%) [58].
The latest International Lipid Expert Panel (ILEP) guidelines from 2023, published simultaneously with the CLEAR Outcomes study, indicate that bempedoic acid is recommended in combination with statins and other lipid-lowering drugs in ASCVD when the LDL-C treatment targets are not met (class of recommendations: I; level: A), in combination with statins and other lipid-lowering drugs in heterozygous familial hypercholesterolaemia (heFH) when the LDL-C treatment targets are not met (class of recommendations: I; level: A); in combination with maximally tolerated statins and other non-statin agents to enable patients with partial statin intolerance to reach therapeutic goals (class of recommendations: I; level: A); in monotherapy or in combination with ezetimibe (FDC) and other non-statin drugs to enable patients with complete statin intolerance to reach their therapeutic goals (class of recommendations: I; level: A) [59]. Based on the available results the ILEP experts also recommended to consider bempedoic acid in primary prevention patients at high and very high cardiovascular risk, who despite optimal, maximally tolerated doses of statins and ezetimbe, have not achieved their LDL-C target (class of recommendation: IIb; level: B); in patients at high and very high cardiovascular risk with elevated hsCRP levels (class of recommendation: IIb; level: B), and in patients with high and very high cardiovascular risk with prediabetes and diabetes to reduce the risk of NOD and improve glycaemia (class of recommendation: IIb; level: B) (Figures 2–4) [59–61].
Obicetrapib
Another drug with interesting properties and lipid disorders management benefits is obicetrapib – a new cholesterol ester transfer protein (CETP) inhibitor (Figure 2). The results of available studies suggest that the drug has a potential to be used in the next step (after statins and ezetimbe) of intensifying lipid-lowering treatment. In a recent phase-2 RCT (ROSE-2 trial) by Ballantyne et al, including 119 patients receiving HIS, it was found that the addition of obicetrapib 10 mg reduced serum LDL-C concentrations by 43.5%, and obicetrapib plus ezetimibe by 63.4%. LDL-C serum concentrations of < 100, < 70, and < 55 mg/dL were achieved by 100%, 93.5%, and 87.1%, respectively, of patients taking the combination. Moreover, obicetrapib and its combination with ezetimibe significantly reduced concentrations of non-HDL-C, apoB, and total and small LDL particles [62]. The ROSE study also showed obicetrapib treatment to significantly decrease apoB (by up to 30%) and non-HDL-C serum concentration (by up to 44%), and significantly increased HDL-C serum concentration (by up to 165%) [63]. Based on the available data obicetrapib (10 mg) can be also a great adding value for patients with elevated levels of Lp(a) with the possibility to reduce it by up to 50% [63, 64]. Additionally, the use of CETP inhibitors, including obicetrapib, reduces the risk of new onset diabetes (RR = 0.84; 95% CI: 0.78–0.91), which means that it can be considered in patients with metabolic disturbances and the risk of diabetes or existing diabetes [65, 66]. Ongoing phase-2 and phase-3 trials – OCEAN (NCT04770389; phase-2 study on the combination of obicetrapib and ezetimibe), BROOKLYN (NCT05425745; phase 3, HeFH), BROADWAY (NCT05142722; phase 3, HeFH and/or ASCVD), TANDEM (NCT06005597; phase 3, HeFH and/or ASCVD or multiple ASCVD risk factors), and the cardiovascular outcome trial PREVAIL (NCT05202509) - will address how best to configure obicetrapib in lipid lowering management (Figures 2, 3). It is also worth mentioning the proof-of-concept study on the role of obicetrapib in early Alzheimer’s disease (AD) (NCT05161715, phase 2a), in which it was observed that obicetrapib reduced cerebrospinal fluid (CSF) levels of 24- and 27-hydroxycholesterol, the oxysterols that may be linked to neuroinflammation and Alzheimer’s pathology [67]. The authors also observed that the reduction in 24- and 27-hydroxycholesterol was paired with an 8% improvement in the plasma β-amyloid (Aβ) 42/40 ratio. Based on these results, it is believed that obicetrapib might offer an advancement for a high-risk patient population for AD development (Figures 2–4) [67].
Inclisiran
Inclisiran is a silencing ribonucleic acid (siRNA) that reduces expression of the PCSK9 gene (Figures 2–4). The administration of inclisiran, compared to placebo, allows for a reduction in the serum concentration of LDL-C by 45–55%, triglycerides by 7–13%, Lp(a) by 17–26% and an increase in the concentration of HDL-C in the serum by 3–6% [68, 69]. In a meta-analysis of 3 randomized clinical trials, including 3660 patients with hypercholesterolemia, it was found that the use of inclisiran reduced serum LDL-C levels by 51% and, more importantly, reduced the risk of major cardiovascular events by 24% (RR = 0.76; 95% CI: 0.61–0.92). It also significantly decreased total cholesterol by 37%, apolipoprotein B by 41%, and non-HDL-C by 45% [70]. In the first real world data with inclisiran the observed effectiveness of inclisiran seems to be less than in the RCTs. In the recent RWE analysis Mulder et al. showed that at 3 months, patients who newly started inclisiran showed an LDL-C decrease of 38% (–49; –33%), patients who used statins as co-medication had a higher median LDL-C decrease compared to those without statin use (45% vs. 38 %), however, those who switched from mAbs to inclisiran surprisingly had an increase in LDL-C of 38% (+4; +97%) [71]. At the ESC Congress 2023 in Amsterdam – the results of the ORION-8 study were presented that was aimed to assess the long-term efficacy and safety of inclisiran in patients who entered the open-label extension after completing either ORION-3, ORION-9, ORION-10, or ORION-11. The longest exposure of inclisiran was 6.84 years, and a quarter of patients were exposed to inclisiran for over 4.45 years [72]. Mean LDL-C reduction was 49.4% with even 51% (–52.2; –49.9%) in ASCVD patients and 42.4% in those with ASCVD risk equivalent (–45.0; –39.9%). At the end of study 78.4% of patients achieved the prespecified LDL-C goal with 79.4% of ASCVD patients being at the goal of < 70 mg/dl (1.8 mmol/l) and 74.3% of individuals with ASCVD risk equivalent at the goal of < 100 mg/dl (2.5 mmol/l) [72]. In the recently published ORION-5 study inclisiran treatment did not reduce LDL-C levels in patients with hoFH (–1.68%; –29.19 to 25.83%; p = 0.90), despite substantial lowering of PCSK9 levels [73]. We need obviously to wait for the results of the ORION-4 (NCT03705234) and the VICTORION-2P (NCT05030428) to see how the efficacy of inclisiran and way of administration may result in reduction of CVD outcomes. In the most recent meta-analysis of 3 randomized clinical trials ORION-9 to 11, including 3713 patients, it was found that inclisiran may reduce the risk of ACS by 32% (0.68; 0.48–0.96) [74].
Lp(a) lowering drugs
Elevated serum Lp(a) concentration is an LDL-C-independent risk factor for ASCVD [64, 75, 76]. Olpasiran, a siRNA that silences the expression of the LPA gene encoding apolipoprotein (a) [apo(a)] (Figures 2, 5), necessary for the synthesis of Lp(a), in a RCT by O’Donoghue et al, including 281 patients with ASCVD, induced an up to even 100% reduction in serum Lp(a) concentrations [77]. A recent study evaluated the association of elevated Lp(a) with CVD outcomes in an observational cohort resembling the OCEAN(a)-Outcomes trial (NCT05581303) main enrolment criteria [78]. In a total of 3142 patients who met the eligibility criteria with median follow-up of 12.2 years, the primary composite outcome occurred more frequently in patients with versus without elevated Lp(a) (by 33%; adjHR = 1.33, 95% CI: 1.12–1.58, p = 0.001) [78]. Zerlasiran (SLN360) has similar effectiveness – it reduces the concentration of Lp(a) in serum by > 95% [79]. Pelacarsen, which is an antisense oligonucleotide that blocks apo(a) synthesis, is slightly less effective. This drug reduces serum Lp(a) concentration by approximately 80–90% [64]. Muvalaplin is the newest drug to lower Lp(a) concentrations in serum and blocks the covalent attachment of apo(a) with the apoB100 moiety of LDL particles (Figures 2, 5). Based on the first presented data the drug reduces the concentration of Lp(a) in serum by 65% [80]. Before we have targeted drugs to effectively lower Lp(a), we need to assess the outcomes in ongoing prospective, randomized clinical trials with these drugs (Figure 5) [76].
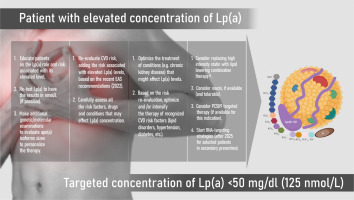
Figure 5
The suggested pathway of management with patients with elevated Lp(a) concentration. Based on Banach M. Eur Heart J Open 2023; 3: oead080 [76] with License Permission No. 5658420691033
Evinacumab
Angiopoietin-like protein 3 (ANGPTL3) is involved in the regulation of triglyceride-enriched lipoproteins (TRLs) ANGPTL3 inhibits lipoprotein lipase (LPL), thereby reducing the clearance of TRLs from the plasma, which include small very-low density lipoproteins and intermediate density lipoproteins [81–83]. Therefore, inhibition of this protein may be important in patients with familial hypercholesterolemia and/or hypertriglyceridemia. Currently, inhibition of the ANGPTL3 protein is possible using evinacumab (an anti-ANGPTL3 monoclonal antibody) or vupanorsen (an antisense oligonucleotide that reduces the synthesis of ANGPTL3 in the liver) (Figure 2), however, for the latter, based on the results from the TaRgeting ANGPTL3 with an aNtiSense oLigonucleotide in AdulTs with dyslipidEmia (TRANSLATE-TIMI 70) trial, in which the magnitude of non-HDL-C and TG reduction observed did not support continuation of the clinical development program for CV risk reduction or severe hipertriglicerydemia (SHTG) the clinical development program was discontinued [84]. Another reason was the safety concerns, as vupanorsen was associated with dose-dependent significant increases in liver fat, and higher doses were associated with elevations in the liver enzymes [84]. Evinacumab reduces the concentration of multiple lipoprotein fractions in serum: LDL-C by 33–49%, triglycerides by 51% and HDL-C by 13%. In patients with homozygous FH, evinacumab has a potential to reduce serum concentrations of total cholesterol by 47%, triglycerides by 55%, non-HDL-C by 50%, apoB by 41%, apoC-III by 84%, Lp(a) by 6% [85]. It appears to do this by both promoting the clearance of TRLs via the VLDL and LDL receptor related protein-1 and reducing hepatic VLDL production and secretion. Evinacumab also proved to be effective and safe in the treatment of children with HoFH – within the follow-up of 24 weeks LDL-C was reduced by mean 48.3%, ApoB by 41.3% non-HDL-C by 48.9%, and total cholesterol by almost 50%. Treatment-emergent adverse events were reported in 10 (71.4%) patients; however, only 2 (14.3%) reported events were considered to be treatment-related (nausea and abdominal pain) [86]. This drug allows for long-term control of the lipid profile in patients with resistant hypercholesterolemia [87]. Despite lack of registration for treating hypertriglyceridemia, evinacumab may be effective (in phase-2 trial in patients with severe hypertriglyceridemia (SHTG; triglycerides > 500 mg/dl) with a history of hospitalization for acute pancreatitis evinacumab reduced triglycerides by mean 27.1%); however, we need to wait for additional data to confirm its efficacy in subjects with SHTG, especially in those with a history of SHTG-associated acute pancreatitis (except familial chylomicronemia [FCS] patients lacking functional LPL) [88, 89].
Olezarsen and volanesorsen
ApoC-III is present on TRL and inhibits LPL, thereby slowing down the clearance of these lipoproteins from plasma. Olezarsen and volanesorsen are antisense oligonucleotides that inhibit apoC-III production in the liver (Figure 2). In patients with hypertriglyceridemia, volanesorsen reduces the serum concentration of triglycerides by 74%, apoB48 by 69%, VLDL-C by 71%, apoC-III by 80%, and increases the serum concentration of HDL-C by 45% and LDL-C by 69%. This drug does not affect the concentration of apoB100 in serum [90]. Based on the recent data from the UK Early Access to Medicines Scheme (EAMS) analysis (drug exposure ranged from 6 to 51 months), volanesorsen therapy among treatment-naive patients resulted in an averaged median 52% reduction (–10.6 mmol/l) from baseline (26.4 mmol/l) in TG levels at 3 months, which were maintained through time points over 15 months of treatment (47–55% reductions). A comparison of pancreatitis event rates found a 74% reduction from the 5-year period before and during Volanesorsen treatment [91]. In patients with hypertriglyceridemia, olezarsen a hepatocyte-targeted, GalNAc-modified antisense oligonucleotide – reduces serum concentrations of triglycerides by 60%, apoC-III by 74%, VLDL-C by 58%, total cholesterol by 8%, non-HDL-C by 20% and apoB by 12–17%, while it increases the serum concentration of: HDL-C by 33–40%, LDL-C by 16% and apoA-I by 14–18% [92]. This drug seems to have very large potential for patients with high and severe hypertriglyceridemia and FCS due to its efficacy and safety and significant ApoB reduction. We await results for outcomes with olezarsen. Multiple randomized, prospective studies are underway, and include: CORE CS5 (NCT05079919, SHTG ≥ 500 mg/dl/5.65 mmol/l), CORE2 CS6 (NCT05552326, SHTG ≥ 500 mg/dl/5.65 mmol/l), CS8 – TIMI 73a (NCT05355402, moderate hypertriglyceridemia ≥ 150 mg/dl/1.69 mmol/l and established or increased risk for ASCVD or SHTG) and ESSENCE CS9 – TIMI 73b (NCT05610280, moderate hypertriglyceridemia ≥ 150 mg/dl/1.69 mmol/l and established or increased risk for ASCVD or SHTG) (Figures 2, 3).
Conclusions and perspectives
It is unacceptable for patients to be treated ineffectively for lipid disorders. We have many classes of LLTs at our disposal. Patients continue to be underdiagnosed and undertreated despite the widespread availability of effective and safe therapies. Physician inertia and failure to treat to risk stratified lipoprotein goals should become issues relegated to the past. Of special importance is ongoing education of patients to increase medication adherence and long-term compliance according to the well-known rule that the more educated the patient, the more adherent the patient. Well-designed media and social-media campaigns (we are failing the fight with anti-statin and anti-LDL-theory movements in social media) should be initiated at the local and national levels. It is also important for medical leaders to establish effective collaboration with government officials in order to increase funding for and availability of dyslipidemia screening programs and drug therapies for patients in need [93–96]. Only then can we cross out the word hypothetically from the statement that lipid disorders patients should be considered as those with rare diseases.