Introduction
Elevated serum low-density lipoprotein cholesterol (LDL-C) is an established risk factor for atherosclerotic cardiovascular disease (CVD) [1–3]. For the general population and those at increased CVD risk, especially at low to moderate risk, the LDL-C reduction by dietary adjustment, and in general lifestyle changes, represents the primary method of CVD risk reduction and therefore deserves special emphasis in the evaluation of lifestyle changes [3, 4]. Dietary adjustments, however, achieve a reduction of LDL-C which is insufficient (usually 10–15%) to reach the recommended LDL-C levels in most cases [4, 5]. The European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) guidelines 2019 as well as some national guidelines recommend lifestyle interventions, which include not only dietary adjustments but also the use of functional foods [4, 6]. The nutraceutical ingredient red yeast rice (RYR) has the higher-level (grade A) recommendation because of its clinically relevant impact on improving lipoprotein profiles [4, 6]. RYR is obtained by the fermentation of rice by the yeast Monascus purpureus, that produces numerous compounds, among which monacolins [7]. Traditional Chinese medicine has been using RYR for a long time; introduction of RYR in food supplements aimed to support the control of abnormal plasma cholesterol levels. Its important role in the lipid-lowering management was also confirmed in the first nutraceuticals guidelines and few expert opinion papers of the International Lipid Expert Panel (ILEP) [7–9]. For individuals who fall into the low-to-moderate CV risk category, changes in lifestyle may be indicated, whereas statin therapy may not (especially in those that response suitably for lifestyle changes) [3, 10]. The guidelines also state that RYR may be considered for subjects with elevated cholesterol levels for whom statin therapy is not yet indicated or in those who are not willing to use statins [6, 9]. The ESC/EAS 2019 guidelines note that clinically relevant cholesterol decreases were reported with RYR amounts containing monacolin K doses of 2.5 mg/day to 10 mg/day [6].
Several RYR-based supplements are available on the market, some contain only RYR, whereas other products contain additional ingredients such as policosanol, astaxanthin, berberine, co-enzyme Q10 (CoQ10) [7, 9, 11, 12]. In fact, such nutraceuticals, that are combination of few effective natural products (nutraceutical polypill/FDC), are mostly recommended [9]. In 2018 the European Food Safety Authority’s (EFSA’s) assessed the RYR use, basing its decisions on selected studies which evaluated supplementation with mono-ingredient products [13]. As a result, as well as a result of numerous new analyses and data from the expert groups, including ILEP that underpinned the EFSA’S opinion [9, 10, 14], the European Commission issued the Regulation (EU) 2022/860 on the use of Monacolins from Red Yeast Rice in Food Supplements, prescribing as condition of use that “individual portion of the product for daily consumption shall provide less than 3 mg of monacolins from red yeast rice” and several mandatory warnings including “Do not exceed consume a daily amount of monacolins from RYR equal or above 3 mg; Contraindicated in pregnancy and breastfeeding, and below 18 years old, and above 70 years old; Contact a physician/HCP in case of side effects; Do not take if already in treatment with lowering-cholesterol drugs or with other products containing RYR” and requiring that the amount of monacolins in each tablet will be now indicated.
The EFSA observed that most reported AEs were musculoskeletal in nature, followed by fatigue, pain, and gastrointestinal (GI) symptoms [13]. Hepatic AEs were also observed to occur in a significant number of patients receiving RYR supplementation, according to the EFSA [13]. The EFSA considered the lactone form of monacolin K to be identical to lovastatin and states that RYR food supplement intake could result in an exposure to monacolin K levels comparable with therapeutic doses of lovastatin [13]. However, considering the selectivity of the data sources used in the report, lack of complete data on the quality production, and lack of rechallenge in the included studies, it was difficult to confirm the causality of the reported AEs with the RYR supplementation [15]. The recent reports, based on the Adverse Event Reporting Systems (AERS), have not also confirmed these adverse effects, indicating low and extremely low prevalence of RYR-related side effects – 0.008% and 0.01% for musculoskeletal and hepatobiliary disorders, respectively [16, 17].
A 2017 analysis of 28 brands of RYR supplements available in the US and the EU showed that 7% provided labelling advising against the concurrent use of statins and that monacolin K content was not included on any of the product labels [18]. In 2 of the analysed products, monacolin K was undetectable, and across the brands that did contain monacolin K, the dose per 1200 mg of RYR ranged from 0.09 mg to 5.48 mg [18]. Moreover, the lack of harmonization among nutrivigilance processes and procedures for food supplement products at the EU level further complicates the EU regulatory landscape of food supplements. As a consequence, the reporting requirements of supplement associated AEs, if they exist, may differ from one EU member state to another. On the contrary, at available controlled trials directly showed that RYR is well tolerated [18–23]. In studies that involved a 6- to 48-week course of Armolipid enhanced RYR supplement, 2.2% of the 1600 treated subjects reported only nonserious AEs, and no life-threatening events were reported. The rates of subjects reporting AEs were not different from placebo [23]. Finally, in the largest available meta-analysis that included more than 8,500 subjects, RYR supplementation was not associated with an increased risk for muscular or non-muscular AEs (which have been observed with statin use) – the same was observed also in individuals with diagnosed statin intolerance; the authors additionally observed significant reduction of SAEs [24].
Based on the abovementioned inconsistency on RYR safety, within the nutrivigilance process, the authors evaluated the safety of a line of RYR food supplements (Meda Pharma; a Viatris Company, Monza, Italy), available since October 2004 to end of 2019, in the postmarketing real-life setting via the company’s nutrivigilance and safety data collection methods [25]. The aim of this analysis is to update the previous evaluation with data collected with the same methodology for other 4 years of real life postmarketing and to update as necessary the safety assessment. Observations on the effect of the age limitation entered by EU Regulation 860/2022 are also drawn.
Material and methods
The methods remain unchanged from previous analysis; however, the time frame is extended to a more recent collection point: 31st Dec 3023 [25]. Briefly, as an example of a proactive approach, by companies in Europe, to report postmarketing information about safety, we report the data from a widely used product, marketed since 1st October 2004, under the trademark Armolipid and Armolipid Plus, manufactured by Meda Pharma, a Viatris Company (Monza, Italy). Evaluated products were well characterized from a quality perspective (including citrinin content), have customer-friendly labelling, and have comparable formulations (RYR content), allowing the analysis and comparison of postmarketing data, while avoiding biases stemming from different contents in the formulations. One tablet of the standard RYR supplement contains RYR (200 mg, the equivalent of monacolin K 2.8 mg), folic acid (0.2 mg), CoQ10 (2 mg), and astaxanthin (0.5 mg), and in some countries, policosanol (10 mg). One tablet of the enhanced RYR supplement contains Berberis aristata extract (588 mg, equivalent to berberine chloride 500 mg), RYR (200 mg, the equivalent of monacolin K 2.8 mg), policosanol (10 mg), folic acid (0.2 mg), CoQ10 (2.0 mg), and astaxanthin (0.5 mg) [26].
The nutrivigilance process was used to monitor the reporting rate and nature of AEs suspected to be associated with the RYR is maintained from previous analysis [25]. An AE was defined as “any untoward medical occurrence” in a consumer while using a food supplement, even when an apparent causal relationship does not necessarily exist. The causal relationship might already have been suspected by the reporter or by the complaining consumer; nevertheless, a causality assessment was performed for all the case reports later in the company’s nutrivigilance process. The AEs described on the food supplement label available to the consumer were considered to be “expected” or “labelled”. Serious AEs were defined as: death; a life-threatening event; an event that required or prolonged hospitalization; or one that resulted in a significantly or persistently incapacitated or disabled state, a birth defect, or a congenital abnormality. Severe events, based on the definition of the European Medicines Agency (EMA) [26, 27], were not considered synonymous with SAEs.
Since product launch in 2004 an internal nutrivigilance database was developed, with increasing global pharmacovigilance-like systems and procedures. The database was validated and implemented to record case reports from worldwide sources. Spontaneous reports originating from healthcare professionals (HCPs), consumers or health authorities were verified as original source reports (i.e., not duplicates), and AEs were coded according to the standard Medical Dictionary for Regulatory Activities. Collected reports included those, in which a correlation between an AE and a food supplement was suspected by the reporter and was later evaluated for causal association (after entry into the database). Additional collected information included reports of deficient efficacy, misuse (e.g., divergence from label instructions), and contraindicated use during pregnancy or breastfeeding. The World Health Organization-Uppsala Monitoring Centre system was selected to guide the evaluation of causality via parameters including time to onset, clinical plausibility, dechallenge, and rechallenge [26]. Cumulative data reported is updated to over a 19-year period (from 1st October 2004 through December 31, 2023), carefully evaluated and correlated with extrapolated consumer exposure to the RYR food supplement line.
Results
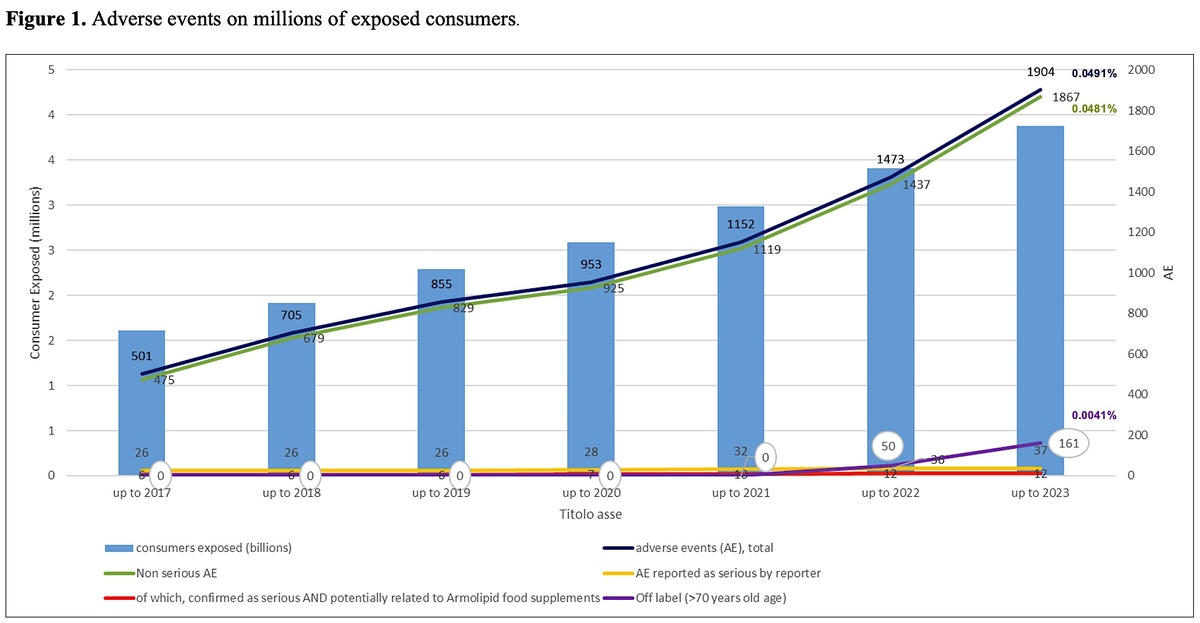
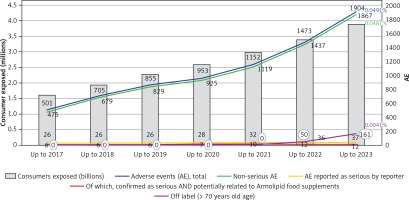
An estimated total of 3,880,865 consumers from both European and extra-EU countries took the recommended 1 tablet per day of the Armolipid RYR-based nutraceutical continuously for 1 year. This number was calculated from an estimated annual exposure based on more than one billion (1,416,515,905) tablets manufactured in the European Union from the time of initial marketing in October 2004 through December 31, 2023, and the recommended dose of 1 tablet per day. The consumer exposure in the previous calculation, from 2004 up to 2019, was 2,287,449 consumers [25]. The current database accumulated 1,186 spontaneous case reports, estimated to equate to 1,186 consumers (542 in the previous analysis), with 1,904 suspected AEs (855 in the previous analysis). Consumers who reported ≥ 1 AEs with respect to use of the RYR changed slightly from 0.0237% of the previous analysis to 0.0306% of the 1-year exposed consumers.
Any-cause adverse events
Also, the percentage of any-cause AEs in association with RYR was basically confirmed, as in the analysis up to 2019 it resulted as 0.0374% and with the updated data is 0.0491%, of the 1-year exposed consumers (Table I). The majority of reported AEs were still nonserious, with a total of 1,867 in 1,904 suspected AEs, which was 98% of all the AEs (97% in the previous analysis) and comprised a 0.0481% prevalence in the exposed consumers. A total of 37 initial SAEs were recorded (including the 26 cases recorded up to 2019 and already presented in previous analysis), received in 28 case reports with ≥ 1 SAEs. The 28 cases related to 28 different consumers, comprising 0.0007% of the exposed consumers. Based on the 37 SAEs, a SAE prevalence equal to 0.001% of exposed consumers was estimated. Upon further evaluation, only 9 of the 37 SAEs qualified as serious reactions (fulfilling the established definition) and reactions, in which the food supplement could not be excluded as the cause. This resulted in a SAE frequency of 0.0002% of exposed consumers. Notedly, this percentage was calculated as 0.0003% in the previous analysis.
Table I
Armolipid line red yeast rice–exposed consumers and adverse events, cumulative data
Gastrointestinal adverse events
GI AEs were the most commonly reported (534/1904), constituting 28% of the AEs and 0.0138% of the RYR-based nutraceutical-exposed consumers. GI-related events included in the 28 case reports of SAEs included the already mentioned in the previous paper reports (diarrhoea, vomiting, nausea with vomiting, lipoedema, intestinal obstruction suspected to be constipation as it did not require hospitalization and resolved with laxative treatment) and 2 new reports mentioning 4 events (diarrhoea haemorrhagic, abdominal pain (2) and retching). Upon evaluation, none of these additional cases reported as serious were confirmed as indeed serious events nor as resulting only from exposure to this nutraceutical.
Musculoskeletal adverse events
Reports of musculoskeletal disorder AEs followed those with GI features in frequency, comprising 241 of the 1904, or 12.6% of the AEs, and 0.0062% of the RYR–exposed consumers. Of the musculoskeletal AEs, 3 were serious in nature; 2 were already discussed in the previous analysis and were associated with noncompliance with the recommendations on the supplement’s label; an additional case, received from ANSES (the French Agency for Food, Environmental and Occupational Health & Safety), of myositis, reported as diagnosis of neuropathic pain and fasciculation and myalgia, was recorded as serious. Myositis is not expected as a serious muscular event, even though muscular pain is expected for statin intolerant users. In this case, available information made reasonable to consider the temporal relation as possible (consumer was taking the product since 22nd Dec and event occurred on 28th Dec same year), dechallenge was suggestive while rechallenge was not reported: in lack of alternative explanation (no information if consumer has a history of statin intolerance) the temporal association and positive dechallenge made suspect a contribution of the product to the event onset, though other causes were not investigated.
No additional reports of rhabdomyolysis were received. Cumulatively, rhabdomyolysis remained reported in 2 consumers (1 involved hospitalization), but in both cases the product’s label warnings were not followed. In the first of these 2 cases, an elderly woman who was taking sertraline and rosuvastatin started the enhanced RYR supplement without seeking medical advice. She developed rhabdomyolysis but recovered after discontinuing the RYR supplement and rosuvastatin. The enhanced RYR supplement label advises against its concomitant use with other hypolipidemic products. The second case involved an unknown-gendered consumer who developed rhabdomyolysis and required hospitalization after having started taking the enhanced RYR supplement without prior medical consultation. This subject had a history of rhabdomyolysis in response to simvastatin, and the enhanced RYR supplement label advises consumers to consult a physician to decrease the risk for musculoskeletal AEs. Therefore, no case of rhabdomyolysis without concomitant or prior statin exposure was detected.
Hepatic adverse events
Hepatic AEs, including reports of transaminase alterations, updated from 26 recorded up to year 2019 to 34 by the end of 2023 of the cumulative 1904 reported AEs, constituting 1.8% of the AEs and a frequency of 0.0009% in the RYR–exposed consumers (0.0011% in the previous paper). Hepatic SAEs on top to the ones discussed in the previous paper were reported in 2 additional consumers. An “acute hepatitis drug-induced” where the date of intake starting of the product was not reported so time to onset could only be assumed as positive, consumer was not taking other drugs or nutraceutical at the time of the event and medical journals, outcome, treatments, investigation of alternative explanations were not provided, not the medical history (including alcohol consumption, previous episodes with drugs, statin past experience) was also not reported; reporting physician assessed the causality as “possible” considering the diagnosis, however it should be noted that the report did not investigated liver transaminases nor biliary stones or other conditions leading to high bilirubin or to cholestasis. The other report was received from the consumer that typed it on Amazon, mentioning that after taking 10 tablets, consumer experienced severe gastrointestinal symptoms, in the following days jaundice and transaminase “at 1000” (colloquial expression to say “very high”), had been hospitalized with a diagnosis of “drug intoxication” and “serious hepatic failure”. Info to clinically interpret the case were scarce in lack of age of user, date of event start, duration, pathologic anamnesis, medical history, hospital medical journals, duration of hospitalization, lab exams, medical treatment, outcome are all unknown.
Of the hepatic SAEs already discussed in the previous analysis [25], causality for one of these cases, wherein the subject had an unremarkable medical history and had also been taking atenolol, levothyroxine, and potassium canrenoate, was considered probable by the reporter. In another case, the causality was considered by the reporting physician to be more likely associated with the amoxicillin/clavulanic acid antibiotic (with liver injury as a known adverse drug reaction) also taken by the consumer. In the other SAE reports, various patterns of presentation (i.e., hepatocellular, cholestatic) were mentioned and the symptoms resolved spontaneously or after treatments of glutathione, cortisone, or ursodeoxycholic acid. The latency range in these reports varied widely, from 1 month to 2 years following RYR supplement intake, and the range of causality included probable (in 1 case), possible (in 2 cases), and unlikely (in 2 cases).
Serious adverse events
The frequency of SAEs was calculated and updated, starting from 1st October 2004 through December 31, 2023. Up to end of 2019, on 2,287,449 exposed consumer 542 reports were received, mentioning 542 consumers and 855 AE, of which 26 SAEs were reported as serious by 21 consumers, confirmed as such 6 SAEs on 6 consumers. Through the end of 2023, exposed consumers totalled 3,880,865, with 1,186 case reports mentioning 1,904 AEs. In total, 37 SAEs were reported as serious by 28 consumers; among them 12 SAEs on 9 consumers were confirmed as serious and where a causal relationship with the RYR products was not excluded, as described in this paper.
Off-label RYR use
A remarkable change was the increase of the off-label use of RYR – from 2004 until 2021, only 13 reports of off label use were recorded (mainly use in combination with other hypolipidemic products); reports increased to 228 up to end of 2023, of which 161 (71%) were reports of exposure to elderly consumer aged 70 years old or more. A reasonable explanation of this increase can be found in the age limitation established by EU Regulation 860/2022, that excludes RYR administration in subjects aged below 70 years. The elderly population or even practitioners used to suggest RYR to people in this age group found out that products they used to take or to suggest were no longer allowed and contacted companies to have information, reporting the exposure in the meanwhile. The use by elderly was considered an off-label report since the date of regulation effectiveness, 22nd June 2022, thus exposure of people aged more ≥ 70 years has been recorded as off label use in the nutrivigilance database from that date, increasing from no case up to 2021 to 161 in just 18 months. None of these reports included SAEs, and only 37 mentioned AEs at all. Notwithstanding the age issue, the safety profile remained stable and satisfactory, confirming the high tolerability of RYR.
Expanding the evaluation up to the past 8 years (Figure 1) confirmed the increases in the number of consumers exposed and in reported AEs. This could be due to increased awareness among HCPs and consumers about the importance of reporting suspected AEs, and possibly as a consequence of the EFSA’s published opinion. Nocebo, or more correctly drucebo effect, cannot be also ruled out as a reason of observed AEs [27, 28]. Some factors that also may influence whether an event is reported include length of time since marketing, market share of the suspect product, publicity of the product, and regulatory actions. The mentioned nutrivigilance system showed improved effectiveness over time, with company’s employees trained to systematically forward any case report to the central office of nutrivigilance. In recent years, reports received from e-sellers like Amazon or via social media increased, likely seen as a rapid and easy way to share one’s opinion.
Discussion
The accurate and meticulous nutrivigilance that was applied to RYR confirmed a very good safety of these products when taken as support to the control of cholesterol in healthy subjects. The 2023-updated analysis presented in this paper showed that the rate of the overall AEs complained during RYR intake was 0.0491% of the 1-year–exposed consumers. The majority of reported AEs remained nonserious, with a total of 1,867 of 1,904 AEs, which was 98% of all AEs and 0.0481 % of the exposed consumers. Of the 28 case reports (which comprised 0.0007% of the accumulated exposed consumers) with ≥ 1 SAEs, 9 qualified as confirmed serious in the nature of the AE and wherein the RYR could not be excluded as the cause, an SAE frequency of 0.0002% of the exposed consumers. Rates evaluated in the previous article are confirmed [25]. It is also worth emphasizing that off label use by subjects older than 70 years old explain the increase of reports since the 2022, when the EU Regulation 860/2022 that contraindicates RYR in this age group came into force. Notably, no SAE was reported with the use of RYR in people older than 70. Among SAEs, hepatic and musculoskeletal events were mainly reported. Rhabdomyolysis or severe acute hepatitis can be associated with several drugs, but literature found their incidence in association with preparations of RYR as extremely rare compared to the more common occurrence with statins. The FDA reporting systems evidenced the very modest frequency of rhabdomyolysis or severe acute hepatitis associated with RYR use [28]. The RYR containing products are confirmed to be well tolerated, as shown by the number of SAEs over the past 8 years. The RYR line’s consumer package leaflets include recommendations regarding dosage, contraindicated conditions (including the concurrent use of drugs for dyslipidemia), and HCP consultation to avoid potential musculoskeletal disorder risk.
Following the constrained use of RYR at doses below 3 mg/day in 2022, a recent paper published the results coming from the analysis of different adverse event reporting systems (FAERS and CAERS) and reviewed the most recent meta-analyses focusing the incidence of muscle symptoms and liver dysfunction [29]. In 10 years up to the end of September 2023, i.e. after the first reported, the frequency of cases with musculoskeletal disorders recorded in the FAERS is very low (0.008%). Accordingly, in the same interval, the CAERS database registered a small number of muscle symptoms and liver dysfunction ascribed to RYR intake. The results of these data reflect the outcome of meta-analyses of RCTs in which RYR administration was not judged in relationship with either muscular adverse events or liver complaints [29].
Considering previous analyses, a surveillance assessment reported a collection of suspected AEs from the Italian Surveillance System of Natural Health Products regarding the potential signs of liver injuries and myopathies [15]. This assessment can only be considered qualitatively. In addition, the authors acknowledged the data analysed had a number of limitations, primarily the unavailability of sales data for RYR food supplements because of their regulatory status (i.e., not reimbursed) [15]. Consequently, such data were not captured in the standard administrative databases to contextualize the number of reported AEs with participant exposure. All supplements that were assessed, except one, contained other natural components than RYR. According to the surveillance authors, the case reports contained limited documentation, lacking information on underlying diseases and suspected concomitant medications. Of importance, such information reported spontaneously from these databases cannot be used to determine incidence rates of AEs [15].
The present results are therefore relevant in demonstrating the importance of a nutrivigilance system applied to food supplements when evaluating the safety of products, once product quality and labelling are established and do not introduce bias. Scientific societies recommend HCPs select products from manufacturers that follow high-level, industry-quality standards [9, 10]. In addition, HCPs are encouraged to report AEs to companies [9, 10]. Companies with standardized products and nutrivigilance systems in place can capture and analyse AE trends for their own products and confirm the products’ labelling or evaluate the need for additional warnings to increase consumer awareness.
This evaluation about nutrivigilance had some limitations and strengths. One of the limitations was the inclusion of only one RYR product line, no other products or formulations have been monitored as they do not report regular nutrivigilance data; thus, these results cannot be extended to other product with less warranty of a pharmaceutical standard quality. These methods of reporting obviously cannot be compared with the pharmacovigilance methods, which are implemented for drugs. However, since no vigilance measures/reporting are required from companies under the current regulations for food supplements/nutraceuticals, we consider this kind of voluntary reporting a good practice, to be widely recommended for monitoring of potential adverse events.
In conclusion, the extended and comprehensive procedure of nutrivigilance, combined with the check of contaminants and high-quality standards of manufacturing confirms to assure the consumer safety. Furthermore, an exhaustive, clear and correct information of physicians, pharmacists and consumers is the necessary warranty for achieving a proper use of these class of products, minimising the risks linked to a poor knowledge of precautions and warnings.
A nutrivigilance system collecting AEs from several sources, including the most modern like Amazon and social media, though often scantly documented and difficult to assess from a clinical perspective, still allows the manufacturer to have the immediate access to the user reports of the safety of their own marketed products.
Following these considerations, and the overview of the updated safety data presented in this paper, RYR line of products is confirmed to be an effective and safe tool in the lipid-lowering management [29–31], provided that consumers comply with the information for use indicated in the label and/or package leaflet and with the healthcare provider recommendations.