Introduction
Endometriosis is defined as the presence of endometrial cells in an abnormal or ectopic site outside the uterine cavity [1]. Common symptoms of endometriosis include painful menstruation and ovulation, severe pelvic cramps, heavy bleeding, pain during sex, urination and intestinal pain, bleeding, and pain between periods [2, 3]. Endometriosis affects about 10–15% of women of childbearing age, 20–50% of women with infertility, and 71–87% of women with chronic pelvic pain [4]. Laparoscopy (gold standard) is the primary method of diagnosing endometriosis. For the treatment of symptomatic endometriosis, hormone therapy and analgesics are used; however, the endometriosis often returns [4, 5]. Therefore, it is important to have a comprehensive and individualized approach to the patient and to find a non-invasive marker. Recently, researchers have become interested in the interaction between the cardiovascular system and endometriosis. Cardiovascular disease (CVD) remains the leading cause of mortality among women, causing 1 in 3 deaths each year, while endometriosis affects 1 in 10 women of childbearing age [6]. This unequivocal epidemiological observation should be taken into account in the context of future cardiovascular studies in women. Cardiovascular diseases in women with endometriosis remain understudied and underdiagnosed. A more detailed study of the interaction of the cardiovascular system with endometriosis is needed to fully understand its clinical significance, basic pathophysiology, possible ways of early diagnosis, and prevention. The study of the interaction of endometriosis with the cardiovascular system may be of great clinical importance [7, 8]. Recent studies have shown that non-coding RNAs (ncRNAs) may contribute to the pathogenesis of endometriosis [9, 10]. ncRNAs are divided into 2 main classes depending on the length: long non-coding RNA (lncRNA) – with a size of more than 200 nucleotides; and microRNAs (miRNAs) – about 20 nucleotides [11]. lncRNAs affect the regulation of the flow process in genetic information. This occurs as a result of modulation of chromatin structure, transcription process, splicing, mRNA stability, mRNA availability, and post-translational changes [12–15]. lncRNAs have interaction domains for DNA, mRNA, miRNA, and proteins defined by nucleotide sequence and secondary structure [16–19]. Literature data indicate that lncRNAs have the potential to affect the development and persistence of endometriosis. This is because inflammation, proliferation, and tissue remodeling are modulated [16]. Recent studies indicate that expression levels of Homeobox transcript antisense RNA (lncRNA HOTAIR) were elevated in endometriosis [20–22]. HOTAIR is a 2.2 kb-long lncRNA that is transcribed from antisense strand of HOXC gene cluster present in chromosome 12 [23]. The literature data indicate that patients with aggressive endometriosis showed higher levels of lncRNA HOTAIR [20]. Zhang et al. showed that the level of expression of lncRNA HOTAIR is elevated in ectopic endometriosis tissues. Exosomal lncRNA HOTAIR can promote angiogenesis and progression of endometriosis via the miR-761/HDAC1 axis [22]. Genetic changes in lncRNA HOTAIR may therefore be one of the risk factors leading to the development of endometriosis. However, despite the above reports of endometriosis regulation by lncRNA HOTAIR, its molecular mechanism has not been fully elucidated.
The aim of this study was to analyse the level of expression of lncRNA HOTAIR in patients with endometriosis and to correlate the findings with both clinical and pathological data and with the risk of endometriosis.
Material and methods
Patients
The material for the study comprised tissue scraps embedded in paraffin blocks from 100 endometriosis patients and from the control group (n = 100). The endometrial capsule was obtained by laparoscopy.
Paraffin blocks came from the archives of the Department of Pathology of the Polish Mother’s Memorial Hospital Research Institute, Lodz. The study group consisted of 100 women operated in the Department of Gynaecology, Oncological Gynaecology and Endometriosis Treatment, Polish Mother’s Memorial Hospital Research Institute in 2010–2015 due to endometriosis. The study included patients with endometriosis without associated diseases.
Endometriosis was confirmed during the operation by intraoperative examination and as a result by histopathological examination. The clinical stage of patients with endometriosis was determined according to the rASRM scale (Revised American Society for Reproductive Medicine classification of endometriosis 1996) [24]. The control group included 100 patients. For women in the control group, a trial abrasion was performed for uterine fibroid. In this group, a histopathological examination revealed normal endometrium and no endometriosis. The control group consisted of hospitalized women without endometriosis.
Clinical and pathological data of patients and controls are presented in Table I. The research presented in the paper has been verified and approved by the Local Ethics Committee of the Polish Mother’s Memorial Hospital Research Institute (No. 88/2022).
Table I
Clinical and pathological data of patients (n = 100) and control group (n = 100)
Assessment of histopathological scanner (3DHistech)
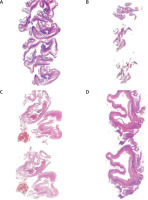
The histological examinations were performed by experienced pathologists using a digital slide scanner and slide viewer software (Case Viewer 2.3, 3DHistech, Budapest, Hungary) (Figure 1). The histological sections were scanned using a Panoramic scanner (3DHistech, Budapest, Hungary), and digital images were obtained. The authors consider that the scanned digital form of a histological section, which can be evaluated using automated software analysis modules, is an independent and objective method to histopathological examination. The study received an internal funding grant from Operational Program Digital Poland MDB-MEDICAL DATA BANK (Grant no. POPC.02.03.01-00-0091/19).
RNA isolation
The High Pure RNA isolation kit (Roche Diagnostics GmbH, Sandhofer Str. 116, 68305 Mannheim, Germany) was used to isolate RNA from formalin-fixed paraffin-embedded tissue (FFPE). The total RNA was used for cDNA synthesis or stored at –80°C until use.
RT-PCR methods
The reverse transcription reaction was carried out using the cDNA synthesis kit Maxima First Strand (Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer’s protocols. The starting material was 500 ng of total RNA. Reverse transcription was carried out under the following conditions: 25°C for 10 min, 50°C for 30 min, and 85°C for 5 min. cDNA samples were stored at –20°C. For the quantitative assessment of lncRNA, the assay used was TaqMan™ Gene Expression Assays (Applied Biosystems, Lincoln Center Dr Foster City, CA, 94404-1128, USA). GAPDH was used as an internal control. qPCR reactions were carried out in a volume of 10 µl, including 10 ng cDNA, 5 µl TaqMan Fast Advanced PCR Master Mix (Applied Biosystems, Lincoln Center Dr Foster City, CA, 94404-1128, USA), and 0.5 µl of appropriate primer (20×).
The sequences of the qRT-PCR primers were as follows: LncRNA HOTAIR, forward primer: 5′-AATAGACATAGGAGAACACTT-3′, reverse primer: 5′-AATCTTAATAGCAGGAGGAA-3′, GAPDH forward primer: 5′-ACAACTTTGGTATCGTGGAAGG-3′, reverse primer: 5′-GCCATCACGCCACAGTTTC-3′. The samples were incubated in a 96-well plate at 95°C for 3 min, followed by 40 cycles of 95°C for 1 s and 60°C for 20 s. The Real-Time PCR reaction was performed in the Mastercycler® ep realplex (Eppendorf, Hamburg, Germany). To determine the relative level of expression, the 2–ΔΔCq method was used.
Statistical analysis
The Shapiro-Wolf test was used to analyse the normality of the distribution. The obtained results were analysed using the χ2 independence test to compare frequency or frequency distributions; the Mann-Whitney U test was used to compare 2 independent groups, while the analysis of multiple mid-rank comparisons for all trials was performed using the Kruskal-Wallis test with the Dunn post-hoc test.
All test results were developed using PQStat v. 1.6.6 (PQStat Software, Poznan, Poland). All statistical tests were carried out at a significance level of α = 0.05.
Results
For this study, the histological preparations were made from archival samples, which were stained with haematoxylin and eosin. The slides were scanned using a panoramic histological scanner (3DHistech, Budapest, Hungary). Endometriosis sites were selected by a panoramic viewer (3DHistech) and used for testing (Figure 1). Histological examinations were performed by 2 experienced pathologists using a digital slide scanner and slide viewer software (Panoramic and Case Viewer 2.3, 3DHistech, Budapest, Hungary).
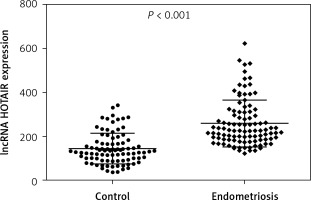
Analysis of lncRNA HOTAIR expression is presented in Table II. As a result of the conducted analyses, a statistically significantly higher expression of HOTAIR in endometriosis preparations was found compared to the expression in the control material (p < 0.0001) (Figure 2).
Table II
lncRNA HOTAIR expression in endometriosis samples and control material
| lncRNA | Test material | Median | Percentile 25 | Percentile 75 | P-valuea |
|---|---|---|---|---|---|
| HOTAIR | Endometriosis | 314.92 | 189.54 | 398.62 | < 0.0001 |
| Control | 189.12 | 96.14 | 256.32 |
Expression analysis according to the clinical characteristics of the patient
The results of the analysis of lncRNA HOTAIR expressions depending on age, parity, and number of spontaneous abortions and body mass index (BMI) of patients are included in Table III. In the case of HOTAIR expression analyses with respect to patients’ age, parity, number of spontaneous abortions, and BMI, no statistically significant relationships were found.
Table III
lncRNA HOTAIR expression in endometriosis samples with respect to age, parity, number of spontaneous abortions, and BMI
| lncRNA | Features | Median | Percentile 25 | Percentile 75 | P-valuea |
|---|---|---|---|---|---|
| HOTAIR | Age | 143.01 | 146.56 | 211.28 | > 0.05 |
| Parity | 113.63 | 189.65 | 214.78 | ||
| Spontaneous abortion | 132.46 | 176.23 | 245.77 | ||
| BMI | 232.32 | 126.56 | 243.56 |
Expression analysis depending on the stage of endometriosis
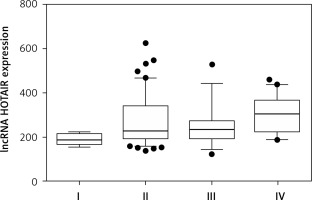
The high expression of HOTAIR was significantly associated (p = 0.0075) with advanced stages of endometriosis (Table IV). With regard to the classification of endometriosis (stage), there was a statistically significant increase in HOTAIR expression in stage IV compared to stages I, II, and III, with p < 0.05 in all 3 cases (Figure 3).
Table IV
lncRNA HOTAIR expression in endometriosis preparations depending on the stage of the disease according to rASRM
| lncRNA | Median | Percentile 25 | Percentile 75 | P-valuea |
|---|---|---|---|---|
| HOTAIR: | ||||
| I | 197.11 | 185.78 | 220.31 | 0.0075 I vs. II > 0.05 I vs. III > 0.05 I vs. IV < 0.05 II vs. III > 0.05 II vs. IV < 0.05 III vs. IV < 0.05 |
| II | 224.66 | 196.81 | 318.55 | |
| III | 230.55 | 192.82 | 252.68 | |
| IV | 338.55 | 234.13 | 398.87 |
Discussion
lncRNAs are known to be critically involved in a wide range of biological processes such as cell cycle regulation, pluripotency, differentiation, and cell death [25–28]. A risk factor leading to endometriosis may be genetic changes in lncRNA HOTAIR [9]. Unfortunately, the relevance of HOTAIR lncRNA in the development and progression of endometriosis remains completely unknown. It is known that lncRNAs HOTAIR can affect many miRNAs. Therefore, it may participate in the development and occurrence of diseases, including endometriosis [21, 29, 30]. Exosomal lncRNA HOTAIR is known to activate endometriosis progression and angiogenesis via the miR-761/HDAC1 axis [22]. lncRNA HOTAIR binds to miR-519b-3p to control the expression level of miR-519b-3p. Decreased expression of HOTAIR lncRNA may increase the level of expression of miR-519b-3p. The consequence of this process is to regulate the ability of endometrial stromal cells to invade and migrate [21]. It is worth noting that the lncRNA pathway HOTAIR/miR-519b-3p/PRRG4 may be a research target and a potential marker in the early diagnosis and treatment of endometriosis. Chang et al. showed that the genetic polymorphisms rs1838169 and rs17720428 in HOTAIR may be risk factors leading to the development of endometriosis. Polymorphisms can affect increased HOTAIR levels [20]. HOTAIR was originally discovered from microarray analysis by Rinn et al. [23]. HOTAIR is located at the HOXC locus on chromosome 12, flanked by HOXC12 and HOXC11. HOTAIR is known to be transcribed in an antisense manner with respect to the HOXC genes. Studies by Rinn et al. showed that siRNA-mediated knockdown of HOTAIR leads to transcriptional activation of HOXD locus genes present in chromosome 2, including HOXD8, HOXD9, HOXD10, and HOXD11 [23]. However, there was no significant effect on the transcription of genes of HOXC clusters present in chromosome 12, where HOTAIR is actually encoded [23]. Suppression of HOTAIR upregulates the levels of HOXA5 and downregulates MMP2 (matrix metallopeptidase 2) and MMP9 (matrix metallopeptidase 9), which are critical players of tumour invasion and metastasis [31]. The alternation of HOXD10 and HOXA5 expressions (a class of genes encoding transcription factors) has been identified as a mechanism of infertility associated with endometriosis [32, 33]. HOXD10 and HOXA5 are key regulators for HOTAIR when promoting endometriosis progression. HOXD10 and HOXA5 are regulated by HOTAIR in advanced endometriosis. These 2 proteins play negative regulatory roles in cell proliferation, migration/invasion, and angiogenesis [34–36]. Lowering these 2 goals by stabilizing HOTAIR and raising in advanced endometriosis can provide a favourable microenvironment for the growth of lesions and spread to other organs. The functional axes HOTAIR/HOXD10 and HOTAIR/HOXA5 can play an important role in regulating the progression of endometriosis. Targeting HOTAIR is a potential strategy for treating endometriosis and inhibiting further malignant transformation.
In our study, we presented an analysis of the expression of lncRNA HOTAIR in patients with endometriosis compared to controls, with the aim of a possible correlation with the risk of the above-mentioned disease. We chose to study the above lncRNA because the literature data on analysis of its level of expression in endometriosis patients are scarce. In the work, we used a modern histopathological scanner for the precise selection of endometriosis tissue for testing. The scanner ensures high precision and confidence in the selection of tissue for research.
Our statistical analysis showed a significant correlation of lncRNA HOTAIR expression levels with endometriosis. The RT-PCR results showed that the average relative expression of lncRNA HOTAIR was much higher in endometriosis patients than in controls. In the presented studies, HOTAIR transpired to be important in terms of interrelation with the stage of the disease. The highest level correlated with grade IV of endometriosis differentiation. Our data are in line with studies that confirm this level of expression in endometriosis [21, 22]. According to literature data, the level of HOTAIR expression in ectopic endometrial tissues was significantly higher than in eutopic endometrial tissues and normal endometrial tissues [21, 22]. The high level of expression of HOTAIR promotes the ability to migrate and invade endometrial stromal cells. This phenomenon is important in the pathogenesis of endometriosis [22]. The results of the analysis of the level of expression of lncRNA HOTAIR presented in our work show the existence of certain associations with endometriosis.
Limitations of the study – it should be noted, however, that the presented research covered a small population (200 people) and further work on much larger study groups is required. The study groups used may be quantitatively unsatisfactory to draw appropriate conclusions. A limitation of the study for clinical practice is that the tools used for detection are not yet very common.
Gene expression testing could be a target for personalized therapy in the future. To date, the process of diagnosing the disease has taken a long time. To detect endometriosis, magnetic resonance imaging or laparoscopy is most often used, thanks to which, in addition to diagnostic values, we gain information on the stage of the disease. However, even using highly invasive procedures, such as laparoscopy, there is no certainty that the endometrial focus will be correctly located. Genetic testing of lncRNA gene expression can shorten the diagnostic pathway. In the future, they can be used to create a minimally invasive test for endometriosis. Thanks to gene expression tests, it will be possible to diagnose the disease earlier, which will allow for an earlier start of treatment.
In conclusion, according to our study, elevated lncRNA HOTAIR expression levels may be associated with endometriosis. The current state of knowledge on lncRNA in endometriosis is limited. Further studies are warranted to further explore this subject.