Introduction
Cancer causes millions of deaths worldwide, and more unfortunately, its incidence in many countries is increasing. Nowadays, surgery, chemotherapy, radiation therapy, and immunotherapy are the four commonly used treatment modalities. For many cancers, surgical interventions are potentially curative; however, many tumors are at an advanced stage that cannot be managed by surgical resection at the time of diagnosis in many clinical scenarios. Chemotherapy, which inhibits multiplication of cancer cells, is the major treatment option for most patients. The efficacy of chemotherapy has been widely documented [1, 2]; however, serious adverse effects associated with the use of chemotherapeutic drugs have also been reported [3–5]. Patients treated with chemotherapeutic drugs can develop drug resistance. For example, a significant number of patients with estrogen receptor-positive breast cancer are found to acquire resistance to tamoxifen [6, 7]. Treatment of patients with cancer by chemotherapy, radiotherapy, and surgery is also challenged by the post-treatment tumor recurrence. It therefore remains imperative to identify new modalities for cancer treatment.
Naturally occurring compounds are rich resources for novel anti-cancer drug candidates. Medicinal herbs containing a bioactive alkaloid called berberine have long been used by medical practitioners in China, Unani, and India to treat malignant diseases [8]. The compound berberine has been shown to display potent anti-cancer effects in vitro and in vivo [9–12], of which the underlying mechanisms are largely attributed to induction of the cell cycle and apoptosis in cancer cells [13–15]. More importantly, berberine is relatively non-toxic to humans compared to chemotherapeutic drugs [16]. All these studies collectively suggest that berberine is a promising drug candidate warranting further in-depth investigations. In the present study, we examined the cytotoxic effects of berberine on a panel of cancer cell lines including oral squamous cell carcinoma, nasopharyngeal carcinoma, breast cancer, cervical carcinoma, and colon cancer. We also deciphered the possible mechanism underlying the cytotoxicity of berberine towards cancer cells.
Material and methods
Cell cultures and reagents
Human oral squamous cell carcinoma Tca8113 cells were obtained from the Key Laboratory for Oral Biomedicine of Ministry of Education, Department of Endodontics, School and Hospital of Stomatology, Wuhan University, China. Human nasopharyngeal carcinoma CNE2 cells, human breast cancer MCF-7 cells, human cervical carcinoma Hela cells, and human colon cancer HT29 cells were purchased from the China Center for Type Culture Collection (CCTCC), Wuhan, China. Tca8113 and HT29 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fisher Scientific Inc., MA, US). Hela and MCF-7 cells were maintained in RPMI-1640 (Thermo Fisher Scientific Inc.), while CNE2 cells were maintained in Minimum Essential Medium with Earle’s Balanced Salts (MEM/EBSS; Thermo Fisher Scientific Inc.). All the culture media were supplemented with 5% fetal bovine serum and 1% streptomycin/penicillin. For the culture of MCF-7 cells, human insulin-like growth factor (1 μg/ml) was also added. All the cell cultures were maintained in a 5% CO2 environment at 37°C.
Berberine hydrochloride and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich Co. LLC (St. Louis, MO, USA). The stock solution of berberine was prepared by dissolving berberine hydrochloride in sterile water and filter-sterilized. 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide (MTT) was purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China).
MTT cell viability assay
The effect of berberine on the viability of various cancer cell lines was examined using MTT assay. Cancer cells were seeded into 96-well plates at a density of 1 × 106 cells/well, and on the next day, were treated with berberine of serial concentration (i.e. 12000, 6000, 3000, 1500, 750, 375, 188, 94, and 47 μM). Incubation was allowed for 48 h. After the incubation, 50 μl of MTT (2 mg/ml) was added. After 3 h, the optical density at a wavelength of 600 nm was detected. The IC50 value of each cancer cell was determined from three independent determinations.
Annexin V staining for apoptosis
Apoptosis of cancer cells after treatment with berberine was studied using annexin V staining (KeyGen Biotech, Nanjing, China). In brief, cancer cells were treated with berberine hydrochloride of concentration equivalent to the respective IC50 for different time points (0, 6, 12, 24, and 36 h). The treated cells were then stained with annexin V-FITC and propidium iodide (PI, 10 μg/ml). The proportion of apoptotic cells (annexin V-positive/PI-negative) was determined using flow cytometry by FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, USA).
Cell cycle analysis
The effect of berberine on the cell cycle of cancer cells was examined by PI staining. Briefly, cancer cells were treated with berberine hydrochloride of concentration equivalent to the respective IC50 for different time points (0, 6, 12, 24, and 36 h). Cancer cells were fixed with ethanol for overnight, treated with RNase A (1 mg/ml), and finally stained with PI (100 μg/ml). PI-stained cells were quantified using flow cytometry by FACSCalibur (Becton Dickinson), with the cell cycle being analyzed by CellQuest and Modfit software.
Quantitative real-time PCR
The expression of BAX and BCL-2 genes after berberine treatment was examined using quantitative real-time PCR. Cancer cell lines were subjected to berberine hydrochloride treatment of a concentration equivalent to the respective IC50 for different time points (0, 6, 12, 24, and 36 h). After treatment, total RNA was isolated from the treated cells using TRIZOL (Thermo Fisher Scientific Inc.). First-strand cDNA was then synthesized from the isolated RNA using Superscript II reverse transcriptase (Thermo Fisher Scientific Inc.). Amplification of BAX and BCL-2 was performed using TOYOBO THUNDERBIRD SYBR qPCR Mix (Osaka, Japan) by the ABI 7500 Real-time System (Thermo Fisher Scientific Inc.) with the following oligonucleotides: H-BCL-2, 5′-CATTGGGAAGTTTCAAATCAGC-3′ (sense) and 5′-CTTTGCATTCTTGGACGAGG-3′ (antisense); H-BAX, 5′-TTGCTTCAGGGTTTCATCCA-3′ (sense) and 5′-CAGCCTTGAGCACCAGTTTG-3′ (antisense). Human actin (sense, 5′- GTCCACCGCAAATGCTTCTA-3′; antisense, 5′- TGCTGTCACCTTCACCGTTC -3′) was also amplified as the internal control. Gene expression of both targets was determined as the expression relative to the control using the comparative cycle threshold method.
Western blotting
The expression of BAX and BCL-2 proteins after berberine treatment was studied by western blotting. Whole cell lysate (40 mg) from cancer cell lines, which were treated with berberine hydrochloride of concentration equivalent to the respective IC50 for different time points (0, 6, 12, 24, and 36 h), was resolved on 10% SDS-polyacrylamide gels and electroblotted on polyvinylidene fluoride membranes (Roche Applied Science, Germany). The blots were blocked overnight with 5% nonfat dry milk and probed with primary antibodies at dilutions recommended by the suppliers. Immunoblots were detected by horseradish peroxidase-conjugated secondary antibody (Pierce) using a chemiluminescence kit (Pierce) and photographed.
Results
Berberine suppressed cancer cell viability
The suppression of cancer cell viability by berberine was studied using MTT viability assay after the cancer cells were incubated with berberine for 48 h. Berberine displayed an in vitro cytotoxic effect on all the tested cell lines. The IC50 of berberine treatment for each cell line was determined and compared (Table I). Colon cancer cell line HT29 was the most sensitive one among the five cell lines tested, with its IC50 measuring 52.37 ±3.45 μM. The IC50 values of the remaining four cell lines were comparable to each other.
Berberine induced G2/M-phase arrest
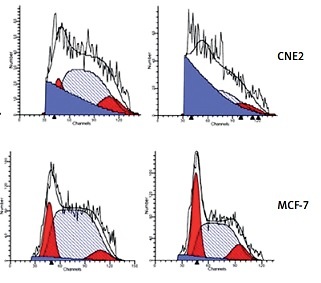
Since the treatment of berberine resulted in suppression in cancer cell viability, we examined whether berberine would affect the cell cycle of different cancer cells by PI staining (Figure 1 A). The proportion of cell cycle phases was determined, and results showed that berberine could induce cell arrest at the G2/M phase (Figure 1 B). For Tca8113 and MCF-7, the maximal arrests were seen after berberine treatment for 12 h. For Hela, CNE2, and HT29, the maxima arrest was observed at 24, 36, and 24 h, respectively.
Figure 1
Berberine arrested cancer cell cycle progression at G2/M phase. A – The cell cycles of cancer cells were analyzed by flow cytometry, with the nuclear DNA of berberine-treated cells labeled with PI. A representative set of histograms from three independent experiments is shown. B – The proportions of G2/M phase of different cancer cells were quantified and compared. Data are presented as the mean ± SD of three independent experiments

Berberine triggered early apoptosis
We also performed annexin V staining to examine the induction of apoptosis in cancer cells by berberine treatment (Figure 2 A). The annexin V-positive cells were quantified and the results demonstrated that apoptosis was increased by berberine treatment in all the cell lines tested in a time-dependent manner (Figure 2 B). To delineate the molecular mechanism by which berberine induced apoptosis in cancer cells, we used western blotting to examine the protein expression of apoptotic regulators BAX and BCL-2 in berberine-treated cancer cell lines (Figure 3 A). It was clearly demonstrated that berberine treatment could increase BAX protein expression; the elevation was accompanied with significant suppression of BCL-2. The effect of berberine on the protein expression of BAX and BCL-2 was time-dependent. To investigate whether berberine would also regulate BAX and BCL-2 at the transcriptional level, real-time PCR was employed to determine the gene expression of BAX and BCL-2 after treatment of different cancer cells with berberine (Figure 3 B). For BAX, its gene expression was substantially elevated by berberine treatment in all cell lines tested except in HT29, while for BCL-2, its gene expression was significantly suppressed in all cell lines tested. Again, the effect of berberine on the gene expression of BAX and BCL-2 was time-dependent.
Figure 2
Berberine induced apoptosis in multiple cancer cells. A – Plots of sorted apoptotic cells. Early apoptotic cells (annexin V+ and PI–) are displayed in the lower right quadrant and late apoptotic cells (annexin V+ and PI+) are shown in the upper right quadrant. A representative set of sorted apoptotic cell plots from three independent experiments is shown. B – The early apoptotic cells were counted by flow cytometry. Data are presented as the mean ± SD of three independent experiments
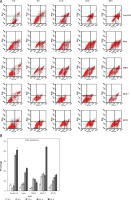
Figure 3
Berberine modulated the expression of BAX and BCL-2 in multiple cancer cells. A – Five cancer cells were exposed to berberine hydrochloride (respective IC50 for each cell line) for 0, 6, 12, 24 and 36 h. The protein levels of BAX and BCL-2 were determined by Western blotting assays. The representative set of western blotting from three independent experiments is shown. B – Relative mRNA expression of BAX at indicated time points. C – Relative mRNA expression of BCL-2 at indicated time points. Data are presented as the mean ± SD of three independent experiments

Discussion
The present study demonstrated the anti-cancer property of berberine on multiple cancer cell lines including oral squamous cell carcinoma, nasopharyngeal carcinoma, breast cancer, cervical carcinoma, and colon cancer. Our in-depth mechanistic studies suggested that the anti-cancer property of berberine was apparently attributed to its ability to trigger apoptosis and to induce cell cycle arrest at G2/M phase. Treatment of cancer remains challenging, despite the recent advents in targeted therapy and immunotherapy. New therapeutic agents for cancer treatment are urgently needed. Over the last decades, in the search of new therapeutic agents, many naturally occurring dietary compounds have been shown to display profound anti-cancer properties with minimal toxicity in various in vitro and animal models [17–19].
Berberine, which is an alkaloid that exists as the major bioactive compound in many medicinal herbs, is one of the naturally occurring compounds that attract intense investigations. The anti-cancer effects of berberine have been characterized, suggesting that the compound could inhibit DNA and protein synthesis and arrested cell cycle progression [20–24]. In line with these studies, we further demonstrated the anti-cancer effect of berberine on different cancer cell lines including Tca8113, Hela, CNE2, MCF-7 and HT29 cells. In these cell lines, berberine prevented cell cycle progression at G2/M phase. The arrest of the cell cycle at G2/M by berberine was also reported in BALB/3T3 cells [25, 26], while in murine L1210 cells, berberine arrested the cell cycle at G0/G1 phase [27]. Our data together with the published findings collectively suggest that the regulation of the cell cycle by berberine would be cell type-dependent.
Induction of apoptosis represents one of the key mechanisms of action of chemotherapy, radiotherapy and immunotherapy [28–30]. Apoptosis is a tightly regulated process involving many anti- and pro-apoptotic proteins. Overexpression of anti-apoptotic members of the Bcl-2 family has been implicated in the chemoresistance of cancers [31–33], whereas high levels of pro-apoptotic proteins, e.g. BAX, promote apoptosis and sensitize tumor cells to various anticancer therapies [19, 20, 34]. In this context, whether cancer cells would be committed to apoptosis partly depends upon the balance between anti- and pro-apoptotic proteins. Our present study clearly showed that berberine treatment could induce apoptosis in multiple cancer cell lines by increasing BAX and decreasing BCL-2 expression. The consistent changes in expression of BAX and BCL-2 indicated that the BAX/BCL-2 signaling pathway may represent a common mechanism of berberine to inhibit the growth of tumors, which is a promising direction for developing strategies in the treatment of various tumors. The increased BAX might override the protective action of BCL-2 on cancer cells; however, the elevation of BAX alone was found not sufficient to initiate apoptosis in the absence of additional stimuli [25]. The berberine-induced apoptosis likely involved other pro-apoptotic pathways or factors yet to be identified.
In conclusion, our results clearly illustrated the anti-cancer action of berberine on multiple cancer cell lines, covering oral squamous cell carcinoma, nasopharyngeal carcinoma, breast cancer, cervical carcinoma, and colon cancer. Advanced tumors of these cancers are difficult to treat, despite the recent advents in new treatment modalities such as immunotherapy. Berberine holds promise as a drug candidate that can be developed into new cancer drugs with minimal toxicity. Our study and findings from other research groups worldwide collectively suggest that berberine can trigger apoptosis and induce cell cycle arrest in cancer cells. Nevertheless, the pharmacologic actions of berberine will require further in-depth investigation in different animal models of cancers.