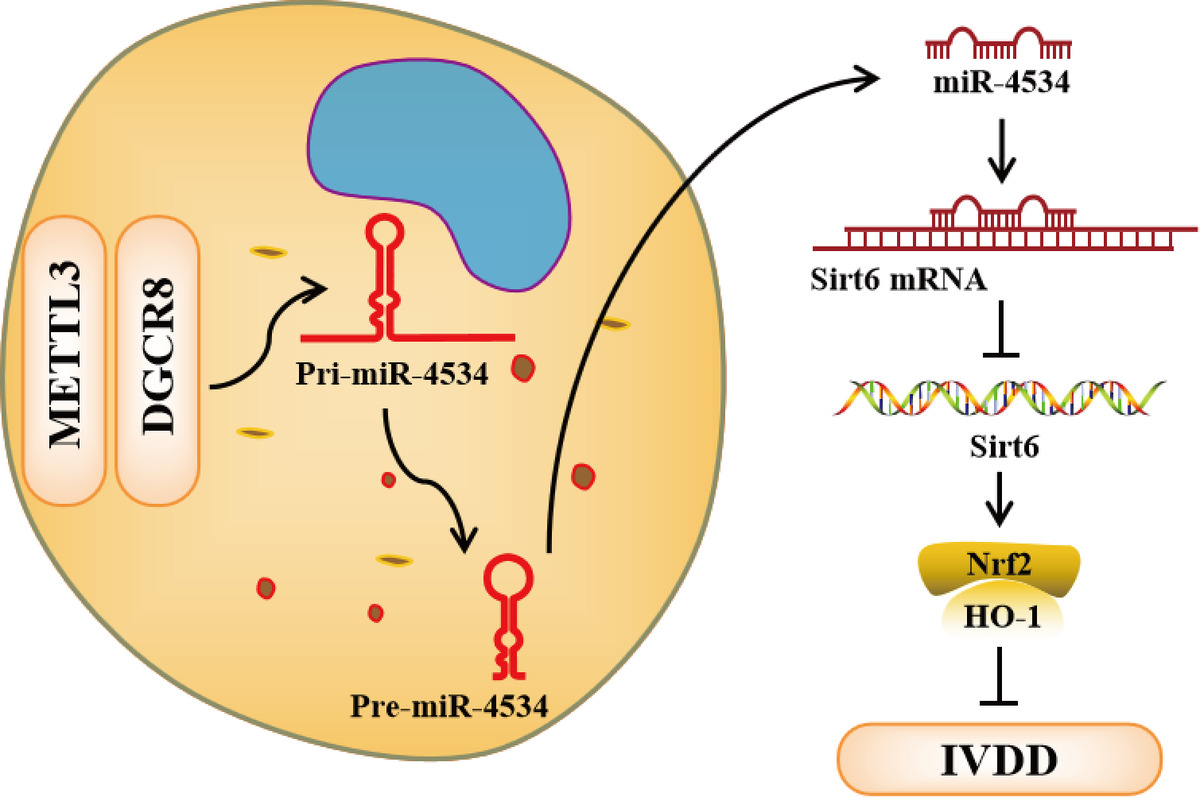
Introduction
Intervertebral disc degeneration (IVDD) significantly contributes to the development of discogenic low back pain, which can have adverse effects on daily functioning and quality of life [1] and may even lead to substantial global disability [2]. Although various treatments aim to alleviate IVDD-related pain, they do not effectively halt the progression of the condition or promote tissue regeneration [2]. Consequently, it is imperative to further investigate the pathogenesis of IVDD and identify more effective therapeutic strategies.
The intervertebral disc comprises three distinct regions: the annulus fibrosus (AF), nucleus pulposus (NP), and cartilaginous endplates [3], among which the degeneration of NP is a major contributor to IVDD and low back pain [4]. Nucleus pulposus cells (NPCs) constitute the primary cell type within the NP and are responsible for synthesizing type II collagen and aggrecan, and their depletion represents a hallmark of IVDD [5]. It has been demonstrated that NPCs, surrounded by extracellular matrix (ECM), are critical in regulating IVDD [6], and the abnormal apoptosis and ECM degradation of NPCs represent the major molecular alterations observed during IVDD [7]. Nevertheless, the precise mechanisms underlying these molecular changes remain unclear.
The role of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream-regulated protein, heme oxygenase-1 (HO-1), has gained significant attention for understanding the pathogenesis of IVDD [8]. As IVDD progresses, there is a significant decrease in the expression levels of both Nrf2 and HO-1, contributing to the progression of the condition [9]. Furthermore, it is important to highlight that HO-1 expression can be regulated at the transcriptional level by Nrf2 [10], and activation of the Nrf2/HO-1 pathway holds promise for mitigating NPC dysfunction in IVDD [11]. Additionally, the Nrf2/HO-1 pathway plays a pivotal role in regulating NPC apoptosis and ECM degradation in IVDD [8]. Consequently, the identification of molecules capable of modulating the Nrf2/HO-1 pathway in IVDD is of paramount significance in advancing our understanding of IVDD’s pathogenesis.
Sirtuin 6 (Sirt6) has garnered significant attention due to its role in counteracting aging and age-related diseases [12]. Notably, its expression has been found to be downregulated in the context of IVDD [13], suggesting a close association with IVDD pathology [14]. Additionally, Sirt6 has been reported to promote the Nrf2/HO-1 pathway and is linked to cellular apoptosis [15]. Consequently, we hypothesize that Sirt6 may act as a mediator of the Nrf2/HO-1 pathway, influencing the apoptosis of NPCs and the degradation of ECM in IVDD, underscoring the importance of exploring the regulation of Sirt6 expression in IVDD.
MicroRNAs (miRNAs), recognized as pivotal regulators of gene expression, have emerged as pivotal contributors to IVDD progression by modulating NPC apoptosis and ECM degradation [16]. Based on this, we hypothesized the existence of at least one miRNA responsible for regulating Sirt6 gene expression. Recently, miR-4534 was identified as upregulated in IVDD-affected NP tissues [17], although its role in IVDD remains unclear. Thus, we propose that the abnormal expression of miR-4534 could play a regulatory role in Sirt6 expression in IVDD.
Mounting evidence supports the critical role of N6-methyladenosine (m6A) modification in governing miRNA biosynthesis [18]. Furthermore, methyltransferase-like 3 (METTL3), an enzyme responsible for catalyzing RNA m6A modification, has been observed to be upregulated in IVDD [19] and associated with the regulation of miRNA maturation [20]. These findings have led us to hypothesize that METTL3-mediated m6A modification may be involved in the regulation of miR-4534 maturation and potentially contribute to the upregulation of miR-4534 in IVDD.
Based on the aforementioned findings, we designed this study to enhance our understanding of the mechanisms governing the regulation of NPC apoptosis and ECM degradation in IVDD through the Nrf2/HO-1 pathway. The research could provide insights into the complex molecular processes involved in NPC apoptosis and ECM degradation. Additionally, we aimed to clarify the functional association between METTL3, miR-4534, Sirt6 and the Nrf2/HO-1 pathway in IVDD. Overall, this research study provides insights into uncovering potentially novel therapeutic targets for mitigating the pathological progression of IVDD and relieving associated low back pain.
Material and methods
Collection of clinical samples
After obtaining informed consent, we collected NP tissues from 25 patients undergoing surgery for degenerative disc diseases (IVDD group). Additionally, healthy NP tissues were obtained from 25 patients with lumbar vertebra fractures (LVF group). This study was conducted in accordance with the guidelines and regulations of the Ethics Committee of People’s Hospital of Yangjiang (Ethics Committee approval number: 20230097).
Isolation, culture and treatment of NPCs
NPCs were isolated as previously described [21]. Briefly, after collecting the healthy NP tissues from patients, they were carefully preserved and transported to a highly sterile environment until further use. Subsequently, the tissues were subjected to a series of procedures, including three washes with sterile PBS and incubation with 0.2% collagenase type II (Sigma-Aldrich, USA). The isolation solution was supplemented with 1% penicillin-streptomycin mix (Gibco, USA) and 0.1% fetal bovine serum (Gibco, USA). After 5 days of culture, NPCs were obtained from the fragmented tissues and were further digested using Trypsin-EDTA, then seeded into 25 cm3 culture dishes. For subsequent experiments, only NPC cells from low passages (≤ 4) were used. To create an IVDD cell model, NPCs were exposed to IL-1β (20 ng/ml, 201-LB-005, R&D Systems, USA) for 24 h, following a previously described protocol [22].
Cell transfection
sh-Sirt6, sh-METTL3 (50 nM), pc-METTL3 (0.25 µg), miR-4534 mimic, miR-4534 inhibitor (100 nM) and negative controls were dissolved in DEPC water at the recommended concentrations. Then, Lipofectamine 3000 (Invitrogen, USA) was used to facilitate their transfection into NPCs, and after 48 h of incubation, the efficiency of plasmid transfection using qRT-PCR and western blot analyses was assessed.
Quantitative real-time polymerase chain reaction (qRT-PCR)
The RNeasy Mini Kit (Thermo, USA) was used to extract total RNA, followed by treatment with RNase-free DNase I (Thermo, USA) to remove any genomic DNA contamination. cDNA was synthesized using the cDNA Reverse Transcription Kit (Thermo, USA). qRT-PCR was conducted on a real-time PCR instrument, and GAPDH was used for normalization. The 2-ΔΔCT method was employed to calculate the relative expression of the target genes (Table I for primer sequences).
Table I
Primer sequences used for qRT-PCR
Cell viability assay
Each group of NPCs was plated in 96-well plates at a density of 1 × 104 cells per well and incubated for 48 h, after which 10 µl of CCK-8 reagent was added to each well and allowed to incubate at 37°C with 5% CO2 for 4 h. Subsequently, the absorbance of the samples was measured at 450 nm using a microplate reader.
Flow cytometry
NPCs were harvested using 0.25% trypsin and subsequently stained with an Annexin V-FITC Apoptosis Staining Kit (ab14085, Abcam, UK). After washing twice with PBS, the NPCs were re-suspended in 500 µl of 1x Binding Buffer and incubated with Annexin V-FITC and propidium iodide for 5 min at room temperature in the dark. The stained NPCs were measured using flow cytometry (Beckman Coulter, Fullerton, CA) and their apoptotic ratio was calculated.
Western blot
Protein lysates (30 µg) were extracted from NPCs using RIPA buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 2 mM sodium pyrophosphate, 25 mM β-glycerophosphate, 1 mM EDTA, 1 mM Na3VO4, 0.5 µg/ml leupeptin), followed by separation via SDS-PAGE. After transferring them to PVDF membranes, we blocked them with 5% non-fat milk and then incubated them with primary antibodies at 4°C overnight. Subsequently, the membranes were incubated with secondary antibodies for 1 h at room temperature. We employed an enhanced chemiluminescence reagent for color development, and the grayscale values of each band were quantified using ImageJ. The primary antibodies utilized were anti-Sirt6 (Abcam, ab191385), MMP13 (Abcam, ab39012), MMP3 (Abcam, ab52915), ADAMTS5 (Abcam, ab41037), Nrf2 (Abcam, ab137550), HO-1 (Abcam, ab305290), and GAPDH (Abcam, ab8245) antibodies.
Dual-luciferase reporter assay
The 3′ untranslated region (3′-UTR) fragment of either the wild-type or mutant Sirt6 was incorporated into the pMIR-REPORT luciferase vector (Ambion, USA). We utilized Lipofectamine 3000 (Invitrogen) to co-transfect this vector along with the miR-4534 mimic into the cells. After 48 h of incubation, luciferase activity was quantified using the Dual-Luciferase Reporter Assay System (Promega).
RNA immunoprecipitation (RIP)
Cells transfected with specific plasmids were lysed using RIPA Lysis and Extraction Buffer (Thermo, SA). The extracted protein was incubated with Anti-DGCR8 (Abcam, ab90579) or Anti-IgG (Abcam, ab109489) antibodies overnight at 4°C, followed by a 1-hour pull-down with protein A/G Sepharose (Abcam, ab193262). RNA was purified using Trizol reagent (Invitrogen, USA), and qRT-PCR was employed to measure the enrichment of pri-miR-4534. For m6A-RNA immunoprecipitation (Me-RIP), miR-4534 extracted from cell lysates was used as input to determine the m6A-methylated rate of miR-4534. Antibodies utilized in this experiment included an anti-m6A antibody (Abcam, ab190886) and an anti-IgG antibody (Cell Signaling Technology, #2729). Subsequently, we conducted RNA purification and qRT-PCR experiments using the methods mentioned above.
IVDD animal model
To establish an IVDD animal model, Sprague-Dawley rats (200 g, n = 6 per group) were divided into four experimental groups: control, IVDD, IVDD + sh-NC, and IVDD + sh-METTL3. The control group received intraperitoneal injections of 0.9% saline, while the remaining groups underwent annulus fibrosus (AF) puncture surgery. During this surgery, a 21-gauge needle was inserted into the L3/4 intervertebral discs to a depth of 3.0 mm for 30 s, as previously described [23]. Additionally, rats in the IVDD + sh-NC and IVDD + sh-METTL3 groups received sh-NC or sh-METTL3 (20 nmol/day) via the tail vein. After 4 or 8 weeks, the rats were euthanized, and their intervertebral disc tissues were collected for subsequent analysis. All animal experiments in this study were approved by the Ethics Committee of the People’s Hospital of Yangjiang (Ethics Committee approval number: 20220068).
Hematoxylin-eosin (HE) staining
NP tissues were initially fixed in 4% paraformaldehyde at 4°C for 3 h, then subjected to a series of alcohol/xylene gradients for dehydration, followed by embedding in paraffin and subsequent sectioning into thin slices. These slices were then dewaxed using xylene and dehydrated with ethanol, after which they were stained with hematoxylin solution and eosin for examination and photography using a Leica DM2000 microscope.
TUNEL assay
NP tissues were prepared for the detection of cell apoptosis through a series of steps. Initially, they were fixed and permeabilized, followed by incubation with a TUNEL reaction mixture, which comprised TUNEL enzyme (Roche, 11767305001) and TUNEL label mix (Roche, 11767291910), at 37°C for 1 h. Afterward, the tissues were washed with PBS and stained with DAPI. Images were captured and analyzed using a fluorescence microscope.
Results
MiR-4534 is upregulated in the NP tissues of IVDD and promotes NPC apoptosis and ECM degradation
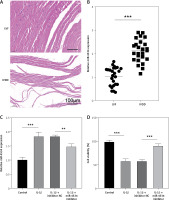
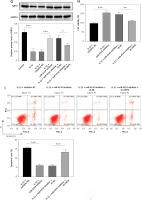
The expression of miR-4534 was found to be elevated in NP tissues affected by IVDD, as previously reported [17]. However, its role in IVDD pathology remained unexplored. To investigate the function of miR-4534 in IVDD, we initially assessed the differential expression of miR-4534 in NP tissues obtained from IVDD and LVF patients. As depicted in Figure 1 A, the IVDD group displayed characteristics such as loose fibrous tissue and the presence of NPC-formed cell clusters, which contrasted with the LVF group. Furthermore, miR-4534 expression was notably higher in the IVDD group (Figure 1 B), suggesting potential involvement of miR-4534 in the development of IVDD. Then, we employed NPCs treated with IL-1β to simulate IVDD in vitro, following a previously established protocol [23] to determine whether miR-4534 is associated with apoptosis and ECM degradation in IVDD [7]. Our results demonstrated that miR-4534 expression increased in NPCs treated with IL-1β but could be downregulated when using a miR-4534 inhibitor (Figure 1 C). Furthermore, IL-1β treatment significantly inhibited cell viability and increased apoptosis in NPCs. These effects were mitigated when miR-4534 was inhibited (Figures 1 D, E). Additionally, IL-1β treatment led to a significant reduction in collagen II expression while elevating the levels of MMP-3, MMP-13, and ADAMTS5 proteins (known contributors to ECM degradation [24]), and these effects could be reversed when miR-4534 was inhibited (Figure 1 F). These findings indicated that miR-4534 is increased in IVDD-affected NP tissues and can promote NPC apoptosis as well as ECM degradation.
Figure 1
MiR-4534 is upregulated in the NP tissues of IVDD and promotes NPC apoptosis and ECM degradation. A – HE staining was used to examine the morphology of NP tissues. B – qRT-PCR was performed to determine the miR-4534 expression in NP tissues from patients with IVDD or LVF (n = 25). C – qRT-PCR was conducted to quantify miR-4534 expression in NPCs under IL-1β or miR-4534 inhibitor treatment. D – The viability of NPCs with IL-1β or miR-4534 inhibitor treatment was assessed using CCK-8. E – The apoptosis of NPCs with IL-β or miR-4534 inhibitor treatment was analyzed by flow cytometry. F – Western blotting was conducted to examine collagen II, MMP-3, MMP-13, and ADAMTS5 protein levels in NPCs with IL-1β or miR-4534 inhibitor treatment. *P < 0.05, **p < 0.01, ***p < 0.001
MiR-4534 promotes NPCs apoptosis and ECM degradation by inhibiting Sirt6 expression
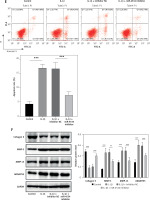
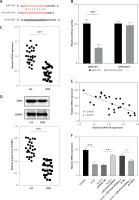
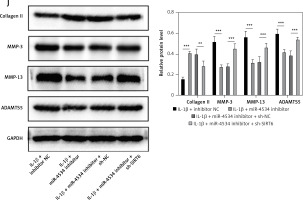
Building on the aforementioned results, this study further investigated the mechanism by which miR-4534 promotes NPC apoptosis and ECM degradation in the context of IVDD. Recent research has revealed that activation of the Sirt6 pathway can rejuvenate senescent NPCs and mitigate IVDD [25]. Thus, we aimed to investigate whether there exists a connection between miR-4534 and Sirt6. Computational prediction using TargetScan 3.1 (https://www.targetscan.org/) indicated that miR-4534 may have binding affinity for Sirt6 (Figure 2 A), which was subsequently confirmed through dual-luciferase reporter assays (Figure 2 B), substantiating the interaction between miR-4534 and Sirt6. Furthermore, our results revealed downregulation of Sirt6 expression in NP tissues obtained from IVDD patients (Figures 2 C, D), and we observed an inverse correlation between miR-4534 and Sirt6 mRNA levels (Figure 2 E). Functional investigations conducted on the IVDD cell model demonstrated that IL-1β treatment decreased Sirt6 expression, while inhibition of miR-4534 increased Sirt6 expression. However, this effect was partially reversed when Sirt6 was simultaneously knocked down (Figures 2 F, G). Inhibition of miR-4534 reversed the IL-1β-induced decline in cell viability and increased apoptosis. Simultaneous knockdown of Sirt6 further mitigated the decline in cell viability caused by IL-1β and exacerbated the IL-1β-induced increase in cell apoptosis (Figures 2 H, I). Additionally, inhibition of miR-4534 partially reversed IL-1β-induced ECM degradation, but this effect was abrogated when Sirt6 was simultaneously knocked down (Figure 2 J). Overall, these findings provide compelling evidence that miR-4534 promotes NPC apoptosis and ECM degradation by suppressing Sirt6 expression.
Figure 2
MiR-4534 promotes NPC apoptosis and ECM degradation by inhibiting Sirt6 expression. A – The interaction between miR-4534 and Sirt6 predicted by TargetScan. B – The binding interaction of miR-4534 and Sirt6 was verified using dual luciferase reporter assay. C – qRT-PCR was performed to measure Sirt6 mRNA levels in NP tissues (n = 25). D – Western blotting was conducted to examine Sirt6 in NP tissues (n = 25). E – Pearson correlation analysis was performed to analyze the relationship between miR-4534 and Sirt6 in IVDD. F – qRT-PCR was performed to assess the expression of Sirt6 mRNA in NPCs under IL-1β, miR-4534 inhibitor or Sirt6 knockdown treatment. G – Western blotting was employed to assess Sirt6 in NPCs under IL-1β, miR-4534 inhibitor or Sirt6 knockdown treatment. H – Cell viability of NPCs under IL-1β, miR-4534 inhibitor or Sirt6 knockdown treatment was evaluated by CCK-8. I – Flow cytometry was performed to assess the apoptosis of NPCs under IL-1β, miR-4534 inhibitor or Sirt6 knockdown treatment. J – Western blotting was utilized to examine collagen II, MMP-3, MMP-13, and ADAMTS5 proteins in NPCs under IL-1β, miR-4534 inhibitor or Sirt6 knockdown treatment. *P < 0.05, **p < 0.01, ***p < 0.001
MiR-4534 inactivates the Nrf2/HO-1 pathway by downregulating Sirt6 expression
Previous studies have established that Sirt6 is involved in mediating the Nrf2/HO-1 pathway, a pathway closely linked to cell apoptosis and ECM degradation [26]. Therefore, this study aimed to investigate whether miR-4534 regulates the Nrf2/HO-1 pathway through its interaction with Sirt6 in the context of IVDD. Our results revealed downregulation of Nrf2 and HO-1 expression (Figures 3 A–C) and a positive correlation between Sirt6 and the Nrf2/HO-1 pathway in NP tissues obtained from IVDD patients (Figures 3 D, E). Furthermore, IL-1β treatment led to a reduction in the levels of Nrf2 and HO-1 proteins, resulting in inhibition of Nrf2/HO-1 pathway activation. However, the inhibition of miR-4534 effectively activated the Nrf2/HO-1 pathway, while the knockdown of Sirt6 partially counteracted this effect (Figure 3 F). These findings suggest that miR-4534 can inhibit the Nrf2/HO-1 pathway by suppressing Sirt6 expression in the context of IVDD.
Figure 3
MiR-4534 inactivates the Nrf2/HO-1 pathway by downregulating Sirt6 expression. A–C – Western blotting was utilized to examine Nrf2 and HO-1 proteins in NP tissues (n = 25). D, E – Pearson correlation analysis was performed to analyze Sirt6 and Nrf2/HO-1 in IVDD. F – Western blotting was utilized to examine Nrf2 and HO-1 proteins in NPCs under IL-1β, miR-4534 inhibitor or Sirt6 knockdown treatment. *P < 0.05, **p < 0.01, ***p < 0.001
MiR-4534 promotes NPC apoptosis and ECM degradation via the Sirt6/Nrf2/HO-1 pathway
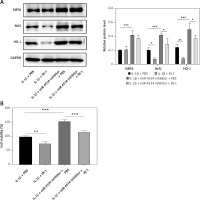
Functional experiments were conducted to investigate whether miR-4534, through its impact on the Sirt6/Nrf2/HO-1 pathway, influences cell apoptosis and ECM degradation. As shown in Figure 4 A, the Nrf2/HO-1 pathway was effectively blocked by IN-1 (HY-146971, MCE, USA), with no significant impact on Sirt6 protein expression. Additionally, the IL-1β-induced downregulation of Nrf2 and HO-1 was further suppressed when the Nrf2/HO-1 pathway was blocked. However, the knockdown of miR-4534 reversed the IL-1β-induced decline in Nrf2 and HO-1 levels. Remarkably, IN-1 treatment further reversed the effect of miR-4534 knockdown on the Nrf2/HO-1 pathway. Furthermore, treatment with IN-1 exacerbated the IL-1β-induced decline in cell viability and increase in apoptosis, whereas it reversed the cell viability increase and apoptosis decrease induced by miR-4534 knockdown (Figures 4 B, C). Additionally, IN-1 treatment promoted the ECM degradation induced by IL-1β, whereas miR-4534 knockdown counteracted this effect (Figure 4 D). These findings collectively indicate that miR-4534 induces apoptosis and ECM degradation by inhibiting the Sirt6-triggered activation of the Nrf2/HO-1 pathway in NPCs.
Figure 4
MiR-4534 promotes NPC apoptosis and ECM degradation via the Sirt6/Nrf2/HO-1 pathway. A – Western blotting was performed to determine the expression of Sirt6, Nrf2 and HO-1 in NPCs under IL-1β, miR-4534 inhibitor or Nrf2/HO-1 pathway inhibitor treatment. B – CCK-8 assay evaluated the cell viability of NPCs under IL-1β, miR-4534 inhibitor or Nrf2/HO-1 pathway inhibitor treatment. C – Flow cytometry analyzed the apoptosis of NPCs under IL-1β, miR-4534 inhibitor or Nrf2/ HO-1 pathway inhibitor treatment. D – Western blotting was utilized to examine collagen II, MMP-3, MMP-13, and ADAMTS5 protein levels in NPCs under IL-1β, miR-4534 inhibitor or Nrf2/HO-1 pathway inhibitor treatment. *P < 0.05, **p < 0.01, ***p < 0.001
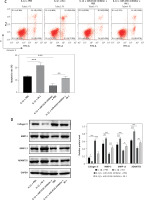
METTL3-mediated m6A epigenetic modification of pri-miR-4534 facilitates the maturation and expression of miR-4534
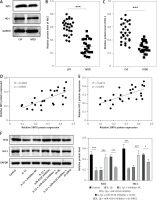
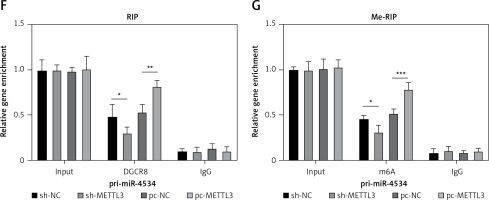
As previously described, miR-4534 plays a crucial role in the regulation of cell apoptosis and ECM degradation. Consequently, its upregulation in IVDD can significantly impact the progression of the disease. However, the mechanisms governing the upstream regulation of this microRNA in IVDD remain elusive. Thus, we assessed the regulatory mechanisms controlling miR-4534 in IVDD. The results revealed that the expression of METTL3 mRNA and protein was significantly upregulated in NP tissues affected by IVDD (Figures 5 A, B). Moreover, METTL3 expression exhibited a positive correlation with that of miR-4534 (Figure 5 C). Subsequently, we successfully generated NPCs with METTL3 knockdown and overexpression, and our findings demonstrated a positive correlation between METTL3 and miR-4534 (Figures 5 D, E). Furthermore, we assessed the enrichment of DGCR8 and m6A antibodies on pri-miR-4534 and observed that it was significantly reduced upon METTL3 knockdown but increased following METTL3 overexpression (Figures 5 F, G). This study provides evidence that the m6A epigenetic modification of pri-miR-4534, mediated by METTL3, promotes the maturation and expression of miR-4534 in the context of IVDD.
Figure 5
METTL3-mediated m6A epigenetic modification of pri-miR-4534 facilitates the maturation and expression of miR-4534. A – qRT-PCR measured METTL3 mRNA expression in NP tissues from patients with IVDD or LVF (n = 25). B – Western blot was performed to determine METTL3 expression in NP tissues (n = 25). C – Pearson correlation analysis investigated the relationship of METTL3 and miR-4534 in IVDD. D – Western blot was utilized to evaluate METTL3 in NPCs. E – qRT-PCR measured miR-4534 expression in NPCs. F, G – RIP and Me-RIP were used to detect pri-miR-4534. *P < 0.05, **p < 0.01, ***p < 0.001

METTL3 upregulates miR-4534 to suppress Sirt6-mediated Nrf2/HO-1 signaling, enhancing apoptosis and ECM degradation of NPCs
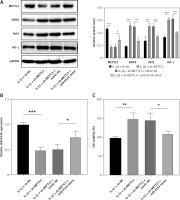
Further investigations were conducted to elucidate whether METTL3 mediates the regulation of the Sirt6/Nrf2/HO-1 pathway by miR-4534. As shown in Figure 6 A, METTL3 protein levels were significantly decreased, while Sirt6, Nrf2, and HO-1 protein levels were enhanced in METTL3 knockdown NPCs treated with IL-1β. However, these effects were partially reversed when miR-4534 was overexpressed. Additionally, Figure 6 B demonstrates the downregulation of miR-4534 in METTL3 knockdown NPCs treated with IL-1β, which was subsequently reversed upon miR-4534 overexpression. Furthermore, cell viability notably increased, and cell apoptosis significantly decreased in METTL3 knockdown NPCs treated with IL-1β, and these effects were reversed when miR-4534 was overexpressed (Figures 6 C, D). Moreover, METTL3 knockdown inhibited IL-1β-induced ECM degradation, which was partially abrogated after the overexpression of miR-4534 (Figure 6 E). The results of this investigation confirm that METTL3 upregulates miR-4534, which in turn inhibits the Sirt6-mediated Nrf2/HO-1 pathway, thereby promoting the apoptosis and ECM degradation of NPCs.
Figure 6
METTL3 upregulates miR-4534 to suppress Sirt6-mediated Nrf2/HO-1 signaling, enhancing apoptosis and ECM degradation of NPCs. A – Western blot analyzed METTL3, Sirt6, Nrf2 and HO-1 protein levels in NPCs under IL-1β, sh-METTL3 or miR-4534 mimic treatment. B – qRT-PCR measured miR-4534 expression in NPCs under IL-1β, sh-METTL3 or miR-4534 mimic treatment. C – CCK-8 evaluated the cell viability of NPCs under IL-1β, sh-METTL3 or miR-4534 mimic treatment. D – Flow cytometry analyzed the apoptosis of NPCs under IL-1β, sh-METTL3 or miR-4534 mimic treatment. E – Western blotting was performed to examine collagen II, MMP-3, MMP-13, and ADAMTS5 protein levels in NPCs under IL-1β, sh-METTL3 or miR-4534 mimic treatment. *P < 0.05, **p < 0.01, ***p < 0.001
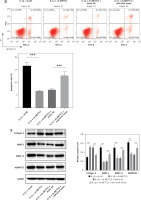
Knockdown of METTL3 alleviates IVDD progression
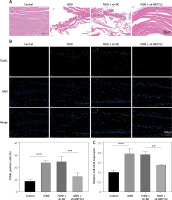
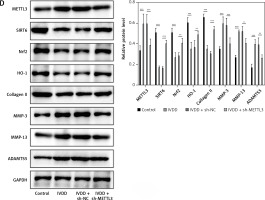
To further explore potential therapeutic strategies for IVDD, we examined the efficacy of METTL3 in alleviating the condition. As shown in Figure 7 A, the intervertebral disc in the control group was mostly composed of NP tissue, with NPCs evenly dispersed within the matrix. In contrast, the IVDD group displayed a smaller NP tissue volume, with NPCs aggregated and separated by a proteoglycan matrix, indicating severe degeneration. In addition, METTL3 knockdown effectively reduced NPC degeneration and fibrosis compared to the IVDD group. Further, the IVDD group exhibited significantly elevated NPC apoptosis, while METTL3 knockdown notably decreased apoptosis among NPCs (Figure 7 B). Notably, Figure 7 C reveals that miR-4534 was upregulated in NP tissues from IVDD rats, whereas its expression was significantly downregulated when METTL3 was knocked down. Moreover, the expression levels of METTL3, MMP-3, MMP-13, and ADAMTS5 proteins were upregulated, while Sirt6, Nrf2, HO-1, and collagen II proteins were downregulated in NP tissues from IVDD rats. These changes were partially reversed when METTL3 was knocked down (Figure 7 D). Collectively, these findings suggest that the knockdown of METTL3 can alleviate the progression of IVDD, indicating that targeting METTL3 may hold promise as a potential therapeutic approach for IVDD.
Figure 7
Knockdown of METTL3 alleviates IVDD progression. A – HE staining was performed to evaluate the morphology of NP tissues. B – TUNEL assay detected cell apoptosis of NP tissues. C – qRT-PCR measured miR-4534 expression in NP tissues. D – Western blot analyzed METTL3, Sirt6, Nrf2, HO-1, collagen II, MMP-3, MMP-13, and ADAMTS5 protein levels in NP tissues. N = 6, *P < 0.05, **p < 0.01, ***p < 0.001
Discussion
IVDD is a complex and chronic condition affecting the spine, characterized by significant pain and disability. Its pathogenesis involves multiple factors, including the apoptosis of NPCs, ECM degradation, and various genetic and environmental factors [27]. In this study, we discovered that the maturation of miR-4534, mediated by METTL3, plays a pivotal role in promoting IVDD progression by targeting the Sirt6-mediated Nrf2/HO-1 pathway. These findings underscore the significance of miR-4534 as a key factor in IVDD and suggest that therapeutic interventions targeting this microRNA may offer a highly effective approach for treating the condition. Furthermore, modulating the upstream or downstream regulators of miR-4534, such as METTL3, Sirt6, and the Nrf2/HO-1 pathway, may also hold promise as effective strategies for IVDD treatment.
N6-methyladenosine (m6A) is a prevalent posttranscriptional RNA modification found in eukaryotic cells, and it plays a crucial role in various biological and pathological processes [28]. This modification process is mediated by “writers”, which comprise a methyltransferase complex responsible for adding a methyl group to the N6 site of adenine within the RRACU sequence motif, while “erasers” in the form of demethylases are involved in reversing this modification [29]. Importantly, in IVDD, m6A modification plays a pivotal role, and the key methyltransferase responsible for m6A modification, METTL3, is upregulated in IVDD [19]. Herein, we confirmed the upregulation of METTL3 in IVDD and its role in promoting the maturation of pri-miR-4534, leading to upregulation of miR-4534 in NPCs. These findings align with the results of a study by Gao et al., which demonstrated that METTL3 promotes the maturation of miR-143-3p and contributes to IVDD [20]. These results collectively suggest that inhibiting METTL3 could potentially be a successful therapeutic approach for treating IVDD. They provide evidence that the abnormal upregulation of METTL3 is a factor that exacerbates the progression of IVDD, and its inhibition may offer an effective strategy to alleviate the symptoms of this disorder.
MiRNA was identified as a key regulator of gene expression and can regulate cell proliferation, development, and metabolism by acting on other genes [16]. It has been determined that certain miRNAs are closely related to the pathogenesis of IVDD, such as miR-138-5p [30], miR-141 [31], and miR-96 [32], predominantly by promoting the apoptosis of NPCs. Additionally, some miRNAs have been found to promote ECM degradation of NPCs to impact IVDD progression, such as miR-154 [33], miR-665 [34], and miR-7 [35]. In this study, we found upregulation of miR-4534 in IVDD, which contributes to ECM degradation and apoptosis of NPCs by inhibiting Sirt6 expression. Although Liu et al. previously determined that miR-4534 is upregulated in IVDD tissues [17], they did not investigate its function and mechanism in IVDD. Here, we are the first to determine that miR-4534 is regulated by METTL3 through m6A modification and can bind to Sirt6 to inhibit its expression. Sun et al. found that Sirt6 expression is downregulated in IVDD, and its upregulation can improve the senescent phenotype of NPCs [25]. Nevertheless, they did not investigate the downstream signal of Sirt6 mediating NPC dysfunction. In addition, Sirt6 overexpression has been found to suppress senescence and apoptosis of NPCs in a model of IVDD [36]. By modulating the expression of Sirt6, the degenerative changes of the intervertebral disc may be reversed, leading to a significant reduction in pain and improved mobility.
This study further indicated that Sirt6 could mediate the Nrf2/HO-1 pathway and impact IVDD progression by regulating NPC apoptosis and ECM degradation. Previous studies have demonstrated the association between Sirt6 and Nrf2/HO-1. Sirt6 could activate the gene expression of Nrf2 and HO-1, and Sirt6-mediated Nrf2/HO-1 signaling was closely associated with different cell types’ apoptosis [15, 26]. Moreover, Sirt6-mediated Nrf2 signaling was found to regulate ECM degradation in osteoarthritis [37]. Additionally, Sirt6 could also restrain NF-κB signaling to lessen ECM degradation in IVDD [38]. In addition, a recent study revealed that the Sirt6-mediated Nrf2/HO-1 pathway could alleviate cell oxidative stress damage [39]. Thus, potentially METTL3-mediated miR-4534 maturation could be investigated further to determine whether it has a protective effect against oxidative damage in NPCs by modulating the Sirt6-mediated Nrf2/HO-1 pathway in IVDD.
This study has revealed a novel mechanism governing the apoptosis of NPCs and the degradation of ECM in the progression of IVDD and highlights the potential therapeutic significance of targeting miR-4534, METTL3 and SIRT6 in alleviating IVDD. Nevertheless, further research is needed to assess the clinical efficacy of these interventions and to identify additional potential therapeutic targets for IVDD. It is equally important to evaluate the potential side effects associated with targeting these molecules and consider factors such as cost and treatment availability in the clinical context.
In conclusion, this study demonstrates that METTL3-mediated miR-4534 maturation promotes IVDD progression by targeting the Sirt6-mediated Nrf2/HO-1 pathway. It suggests that METTL3, miR-4534 and SIRT6 are promising therapeutic targets for alleviating IVDD.