Introduction
Adjuvant endocrine therapy is a key treatment in early oestrogen receptor positive breast cancer (BC) which has led to significant reductions in relapse and improvement in overall survival [1].
In young women, defined as under the age of 40 at diagnosis, with hormone receptor positive BC, the use of tamoxifen alone remains the standard of care in women at low risk of relapse [2]. This is however different in young women who are at high risk of recurrence. As aromatase inhibitors (AI) alone are contraindicated in premenopausal women, this high-risk group would benefit from the combination of ovarian function suppression (OFS) with either tamoxifen or AI, as it is associated with a significant improvement in outcomes compared to tamoxifen alone [2], with a recent individual patient level data meta-analysis of 4 trials involving pre-menopausal women showing a benefit of AI over tamoxifen in reducing recurrence (absolute risk reduction of 3% at 5 and 10 years), with no difference in mortality [3].
Cardiovascular disease has become one of the most important sources of morbidity and mortality in BC survivors, particularly among older women diagnosed with breast cancer [4]. Understanding the effects of these treatments on the cardiovascular health is critical, as it may inform early preventative measures. A recent systematic review showed that the use of tamoxifen is associated with a lower risk of myocardial infarction and angina compared to AI [5]. This was postulated to be due to a combination of the lipid-lowering effect of tamoxifen and the loss of the oestrogen-mediated protective effect (lipid metabolism, vasodilation and inhibition of development of atherosclerosis) [6] with the use of AI.
The long-term effects of breast cancer and its treatment on the cardiovascular health of young women remain understudied and unclear. Apart from the obvious effect on fertility, the profound suppression of circulating oestradiol associated with endocrine therapy and OFS (either permanently or temporarily through surgical, radiation or medical therapy) could have adverse cardiovascular and metabolic effects in younger patients and potentially increase the risk of cardiovascular disease.
The aim of this systematic review was to evaluate the reported cardiovascular and metabolic adverse effects within randomised phase III trials of OFS with either tamoxifen or AI.
Material and methods
We performed a systematic search of the online databases PubMed and Scopus until March 2022 for studies involving premenopausal women with breast cancer being treated with endocrine therapy (tamoxifen or AI) with or without OFS (with either drug therapy, radiation or surgical oophorectomy) as part of adjuvant therapy.
Search strategy
The search strategy utilised the following keywords: “breast cancer”, “premenopausal women”, “ovarian suppression”, “adjuvant therapy” and “randomised controlled trial”, and we restricted the search to full length articles published in the English language and in peer-reviewed journals. Abstracts were screened for suitability and relevant studies retrieved. In order to identify all studies, references of relevant manuscripts were searched for additional studies not identified from the initial database search. Two reviewers (Y.G. and D.L.) independently performed the search and literature screen, and the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) [7] guidelines for reporting systematic reviews and meta-analyses were used to report the findings.
Inclusion and exclusion criteria
The following inclusion criteria were applied: (1) randomised controlled trial (RCT) involving premenopausal women with early breast cancer; (2) compared endocrine therapy with or without ovarian suppression; and (3) reported cardiovascular and/or metabolic outcomes.
The following exclusion criteria were applied: (1) studies involving post-menopausal women or men with breast cancer; (2) performed in neoadjuvant setting; and (3) reports no cardiovascular adverse events. Studies were selected independently based on the inclusion and exclusion criteria, and any discrepancies were resolved via discussion with a third co-author (GYHL), as shown in Figure 1.
Endpoints
The primary outcome of interest was the occurrence of cardiovascular and/or metabolic risk factor-related adverse events. The criteria used to define these adverse events were also recorded.
Data extraction
Data extraction was conducted by two reviewers (Y.G. and D.L.) independently. Baseline clinic-pathological characteristics, treatment and reported adverse events were extracted.
Statistical analysis
Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were estimated for binary variables using a random-effects model with the method of DerSimonian and Laird [8]. Heterogeneity between individual studies was assessed using the χ2 statistic and quantified with the I 2 statistic. All analyses were performed using RevMan Version 5.3.5 software (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Regression analyses were performed comparing the event rates of different outcomes between treatment groups (tamoxifen with OFS vs. tamoxifen alone; tamoxifen with OFS vs. AI with OFS) with p-values of < 0.05 regarded as statistically significant.
Two co-authors (Y.G. and D.L.) independently assessed the included studies using V.2 of the Cochrane risk-of-bias tool for randomised trials (RoB2) [9], following guidance in the current Cochrane Handbook for Systematic Reviews of Interventions. Any discrepancies between the assessors were resolved via discussion and arbitration with a third co-author (GYHL). The internet-based graphic generating platform Robvis was used to create the ROB plot with the results.
Results
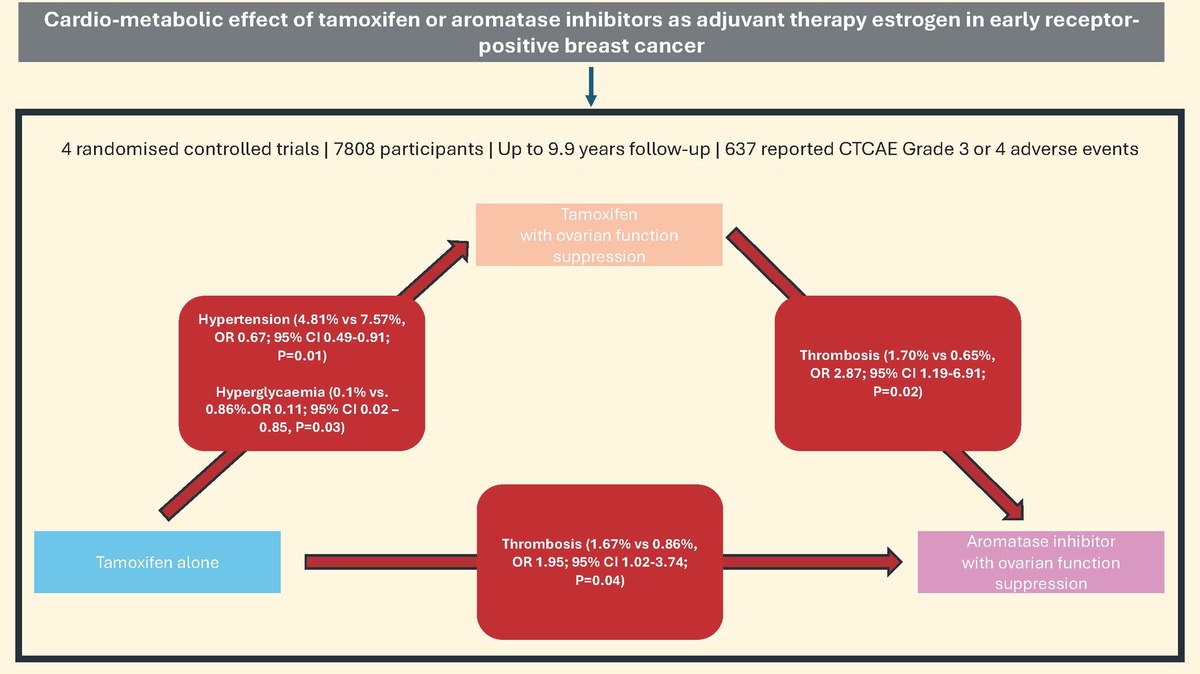
The initial search yielded a total of 167 articles, and after further screening and reviewing of the articles, a total of 3 reports (consisting of data from 4 studies) were included in the final analysis (Figure 1). The four RCTs which met the inclusion criteria were (1) Comparison of Tamoxifen versus Tamoxifen Plus Ovarian Function Suppression in Premenopausal Women with Node-Negative, Hormone Receptor-Positive Breast Cancer (E-3193 INT-0142) [10], (2) Suppression of Ovarian Function Trial (SOFT) [11], (3) Tamoxifen and Exemestane Trial [TEXT] [11] and (4) Austrian Breast and Colorectal Cancer Study Group trial 12 (ABCSG-12) [12]. These studies included a total of 7808 participants after excluding ineligible, withdrawn and loss to follow-up as reported in the original publication. The median duration of follow-up for these studies varies from 47.8 months to 9.9 years (Table I).
Table I
Characteristics of included studies
[i] ABCSG-12 – Austrian Breast and Colorectal Cancer Study Group trial 12, E-3193 INT-0142 – Comparison of Tamoxifen versus Tamoxifen Plus Ovarian Function Suppression in Premenopausal Women with Node-Negative, Hormone Receptor-Positive Breast Cancer, GnRH – gonadotropin-releasing hormone, SOFT – Suppression of Ovarian Function Trial, TEXT – Tamoxifen and Exemestane Trial.
Characteristics of the studies are shown in Table I, and the risk of bias assessment utilising RoB2 [9] is as shown in Supplementary Figure S1. Overall, all the studies were open-label, and adverse events were reported as secondary endpoints. Although all 4 studies used the CTCAE v3.0 criteria [13] (Supplementary Table SI) as standard for reporting adverse outcomes, there was heterogeneity in the cardiovascular and metabolic toxicity reported. Follow-up duration and frequency of follow-up were also not uniform within the studies included. Due to the missing data (domain 3 in the RoB2 tool), all the studies were judged to raise some concerns but were not considered at high risk of bias.
Baseline characteristics and relevant co-morbid conditions
Key clinico-pathological characteristics of the study populations included in the study are summarised in Supplementary Table SII. The median age for all study treatment arms was very similar, at 43 to 45 years of age (range 26 to 56). Of note, no studies reported body mass index or any pre-existing cardiovascular risk factors that were present which would limit the conclusions drawn. None of the studies reported relevant co-morbid conditions such as diabetes, hypertension, atrial fibrillation, coronary artery disease, heart failure, etc.
Exclusion criteria
Regarding relevant cardiovascular disease as an exclusion criterion, SOFT and TEXT used a broad statement: “non-malignant systemic disease that would prevent prolonged follow-up and patients with thrombosis and/or embolism can be included if medically suitable”. E-3193 INT-0142 and ABCSG-12 did not include cardiovascular disease as an exclusion criterion.
Cardiometabolic adverse outcomes and reporting criteria
The four studies reported between two and five relevant cardiovascular or metabolic adverse events, with two reporting two events and the other two five cardiovascular and metabolic adverse events. The reported adverse events were as follows: hypertension and thrombosis reported in 3 out of 4 studies; weight gain, cardiac ischaemia, glucose intolerance and hyperglycaemia in 2 out of 4 studies (Supplementary Table SIII).
The reported adverse events from all studies are as shown in Table II. A total of 637 grade 3 or 4 adverse events were reported, with 414 (65%) cases of documented hypertension (6.9% of reported cohort), 36 (5.7%) reports of weight gain (1.7% of reported cohort), 17 (2.7%) cardiac ischaemic events (0.3% of reported cohort), 93 (14.6%) thrombotic events (1.2% of reported cohort), 42 (6.6%) with glucose intolerance (0.7% of reported cohort) and 35 (5.5%) reported hyperglycaemias (0.6% of reported cohort). 92.7% of the reported adverse events (591 out of 637) were from SOFT and TEXT.
Table II
Reported adverse events in included studies
| Study | Treatment (n) | Outcomes (Grade 3/4) | |||||
|---|---|---|---|---|---|---|---|
| Hypertension n (%) | Weight gain n (%) | Cardiac ischaemia n (%) | Thrombosis n (%) | Glucose intolerance n (%) | Hyperglycaemia n (%) | ||
| ABCSG-12 | Tamoxifen + OFS (451) | NR | 7 (1.6) | NR | 3 (0.7) | NR | NR |
| Tamoxifen + OFS + zoledronic acid (449) | NR | 7 (1.6) | NR | 5 (1.1) | NR | NR | |
| Anastrozole + OFS (453) | NR | 8 (1.8) | NR | 0 (0) | NR | NR | |
| Anastrozole + OFS + zoledronic acid (450) | NR | 4 (0.9) | NR | 1 (0.2) | NR | NR | |
| E-3193, INT-0142 | Tamoxifen alone (n = 167) | 0 (0) | 4 (2.3) | NR | NR | NR | NR |
| Tamoxifen + OFS (n = 170) | 1 (0.6) | 6 (3.5) | NR | NR | NR | NR | |
| SOFT + TEXTa | Tamoxifen alone (n = 1018) | 57 (5.6) | NR | 4 (0.04) | 17 (1.7) | 4 (0.4) | 1 (0.1) |
| Tamoxifen + OFS (n = 2326) | 188 (8.1) | NR | 6 (0.3) | 47 (2.0) | 23 (1.0) | 20 (0.9) | |
| Exemestane + OFS (n = 2317) | 168 (7.3) | NR | 7 (0.3) | 20 (0.9) | 15 (0.6) | 14 (0.6) | |
ABCSG-12 – Austrian Breast and Colorectal Cancer Study Group trial 12, E-3193 INT-0142 – Comparison of Tamoxifen versus Tamoxifen Plus Ovarian Function Suppression in Premenopausal Women with Node-Negative, Hormone Receptor-Positive Breast Cancer, NR – not reported, OFS – ovarian function suppression, SOFT – Suppression of Ovarian Function Trial, TEXT – Tamoxifen and Exemestane Trial.
a Francis et al. study grouped adverse event data from SOFT and TEXT according to treatment group; hence data were combined into three groups: tamoxifen alone (from SOFT), tamoxifen + OFS (combined patients from SOFT and TEXT), exemestane + OFS (combined patients from SOFT and TEXT). Total number for number of patients analysed for safety events differed from original total number of participants due to withdrawals, ineligibility, loss to follow-up, etc.
Regression analysis showed significant differences when comparing the risk of hypertension in the tamoxifen alone group to tamoxifen with OFS (4.81% vs. 7.57%, OR = 0.67; 95% CI: 0.49–0.91; p = 0.01) (Figure 2 A). The tamoxifen alone group showed a lower risk of hyperglycaemia compared to tamoxifen with OFS (0.10% vs. 0.86%, OR = 0.11; 95% CI: 0.02–0.85, p = 0.03) (Figure 2 F).
Figure 2
Forest plots comparing tamoxifen alone versus tamoxifen plus OFS. A – Hypertension*, B – weight gain, C – cardiac ischaemia, D – thrombosis, E – glucose intolerance, F – hyperglycaemia**; p < 0.05

When comparing tamoxifen with OFS and AI with OFS, the former group showed a statistically significant difference in thrombosis events (1.70% vs. 0.65%, OR = 2.87; 95% CI: 1.19–6.9, p = 0.02) (Figure 3 D), whereas other adverse outcomes were not significantly different. In particular, hypertension was not significant (0.86% vs. 0.6%, OR = 1.43; 95% CI: 0.72–2.83; p = 0.31). When comparing tamoxifen alone and AI with OFS, thrombosis was the only adverse event which showed a statistically significant difference, with a higher risk in the tamoxifen alone group (1.67% vs. 0.86%, OR = 1.95; 95% CI: 1.02–3.74, p = 0.04) (Figure 4 C).
Discussion
In this study, we found that endocrine therapy and OFS in premenopausal women increases their risk of developing hypertension, but there appears to be a higher risk of thrombosis in patients on tamoxifen compared to AI. The occurrence of hypertension and/or worsening of blood pressure control accounted for 65% of the total number of adverse events, even with the relatively younger age group being investigated in these trials, highlighting that BC and its treatment do have an impact on the overall cardiovascular risk in these young patients. This was seen when comparing between tamoxifen alone and tamoxifen with OFS, highlighting the impact that OFS and subsequent premature menopause has on development of hypertension over a median period of 9 years within the studies. The early development of hypertension could translate into an increased risk of future cardiovascular disease [14].
In the analyses comparing tamoxifen alone to tamoxifen and OFS, the significant increase in hypertension with the use of OFS could be attributed to the induction of early menopause in young women. The loss of the oestrogen-mediated protective effect [6], which has a role in the increased prevalence of hypertension in women after menopause [7, 15], could contribute to this effect. However, the result of the regression analysis comparing tamoxifen alone and AI with OFS (4.8% vs. 7.3%, OR = 0.76; 95% CI: 0.56–1.03, p = 0.08), which was not significantly different, contradicts this theory. As the analysis relies heavily on the reported outcomes in SOFT and TEXT, there could potentially be bias, which may explain the contradictory results. Similarly, as the cardiovascular risk profile prior to randomisation was not reported, the differences seen could be due to an underlying baseline difference in the groups of patients.
When investigating thrombotic complications (including arterial and venous thrombosis), participants treated with tamoxifen, irrespective of the use of OFS, were observed to have a higher risk than those treated with AI. This would be in keeping with previous meta-analyses showing a higher frequency of venous thromboembolism in patients treated with tamoxifen compared to AI [5]. Tamoxifen is a selective oestrogen receptor modulator which selectively blocks binding of oestrogen to breast cancer cells but induces an oestrogenic effect in other cells [8]. This oestrogenic effect, although it reduces the risk of atherosclerosis, promotes coagulability and hence increases the risk of thrombotic complications [16].
Oestrogen plays a role in the regulation of glucose and metabolism [17] and therefore, with OFS, this could have effects on glucose levels when comparing tamoxifen alone to tamoxifen with OFS. However, the same effect was not seen with glucose intolerance, nor when tamoxifen was compared to AI with OFS. The reported number of hyperglycaemic events was very small in comparison (1 vs. 20) and therefore, although it is statistically significant, should be taken into consideration.
Recent publications from the Pathways Heart Study, one of the largest prospective studies of cardiometabolic risk factors in BC patients, have shown that patients with BC were at higher risk of developing diabetes (all subgroups; hazard ratio (HR) = 1.16; 95% CI: 1.07–1.26), hypertension (only if they had left-sided radiation therapy: HR = 1.11; 95% CI: 1.02–1.21 or endocrine therapy: HR = 1.10; 95% CI: 1.03–1.16) but lower risk of dyslipidaemia (all subgroups: HR = 0.90; 95% CI: 0.93–1.03) over a mean follow-up period of 7 years [18]. This, in combination with our findings, where the median follow-up was about 9 years, informs us that the time in which the treatment would have an impact on the cardiometabolic system may be around 7 to 9 years. Unfortunately, the authors did not differentiate between the type of endocrine therapy within the subgroup analysis to draw further conclusions comparing the two different endocrine therapies. When investigating incident cardiovascular outcomes, women with BC treated with either tamoxifen (HR = 1.80, 95% CI: 1.15–2.82) or AI (HR = 1.43, 95% CI: 1.18–1.73) have shown an elevated risk of cardiovascular death compared to matched controls. Interestingly, there were no significant difference when comparing venous thromboembolism in either of the groups with a matched cohort with BC, although there was a numerical difference in the adjusted HR reported (AI HR = 1.09, 95% CI: 0.78–1.53 vs. tamoxifen HR = 1.69, 95% CI: 0.76–3.77) [19]. Although the mean age of patients in the Pathways Heart Study was around 60 years, which is older than our population being investigated, their findings support and highlight, firstly, the under-recognised risks women with BC face because of their diagnosis and treatment and, secondly, that these cardiometabolic risk factors which they accumulate do translate into an increased risk of cardiovascular morbidity and mortality.
This is the first study to investigate the cardiovascular impact of endocrine therapy in young women with BC. The included studies, although all were open-label, were randomised controlled trials with a modest total number of patients. Baseline characteristics (breast cancer stage and age) were comparable amongst the three groups. One of the main limitations was the absence of reports for the baseline cardiovascular risk profile such as history of hypertension or smoking history. The process of randomisation could have accounted for this, but it is possible that the groups did not have the same cardiovascular risk profile prior to randomisation. This is highly relevant in assessment of future cardiovascular risk, and therefore missing data limit the potential conclusions that may be drawn from existing data. Given the potential impact these therapies may have on cardiovascular risk profile, we recommend that common cardiovascular risk factors (Supplementary Table SIV) form part of the baseline characteristics for future studies. This would allow the dataset to build upon existing cardiovascular risk and truly assess the impact of their treatment.
Similarly, assessment of adverse events in terms of major adverse cardiovascular events (MACE) or the equivalent using CTCAE criteria would be essential to highlight and address these risks within this cohort of patients. The variability and lack of standardisation in the reporting of cardiovascular events within cancer therapy trials are not isolated within the studies we examined. Bonsu et al. reported that nearly 40% of trials did not report any cardiovascular events in the follow-up, and even in those that did, event rates were markedly lower [20]. The latest European Society of Cardiology (ESC) guidelines on cardio-oncology have produced the criteria to define various cancer-therapy related cardiotoxicity and recommended timepoints for follow-up to monitor for cardiotoxic effects of anti-cancer therapy [21]. This could serve as a guide for future trials to standardise the approach towards timely assessment of a less comprehensive but more detailed list of clinically relevant cardiovascular toxicity. CTCAE v3.0, which is currently superseded by v5.0, was used as a reference to classify the adverse events. Although v5.0 has updated some of the definitions to be more in keeping with current cardiovascular societies’ definition of cardiometabolic events, there is still a requirement to standardise the definition to allow better interpretation of these effects in terms of long-term clinical implications.
Second, not all adverse outcomes of interest were reported in all 4 studies (in 3 published papers), and the analyses utilised data from the single main paper reporting both SOFT and TEXT [11]. The events recorded were the total number of events, which could include recurrent events in the same patient, which again limits the conclusions that could be drawn without patient level data. Third, the overall follow-up period remains relatively short to identify cardiovascular events, as the incidence rate is relatively low in young women. Fourth, the addition of zoledronic acid in the treatment groups in the ABCSG-12 study [12] may have confounded the results. Fifth, management of cardiovascular risk factors prior to treatment may vary between studies and could be a source of bias. The limited number and sample size of the included studies also limit the conclusions that can be drawn from our analyses. The use of individual patient level data could be useful to strengthen the analyses and conclusions. Lastly, as the outcomes investigated in this study are associated with cardiovascular risk profiles, their relationship with clinical events such as myocardial infarction or cardiovascular mortality may not be direct.
In conclusion, this study highlights the probable early cardiovascular and metabolic impact of endocrine therapy and OFS in young women with BC. Although the presence or absence of the associated cardiovascular risk may not be the primary reason for the choice of treatment, recognising the potential impact, providing closer monitoring and initiation of early treatment may be beneficial for these young women to prevent future adverse events. Further studies, with particular focus on baseline and development of subsequent cardiovascular risk factors, are needed to understand the underlying impact of these drugs on the cardiovascular health of these young women with BC and assess whether this impact translates to poorer cardiovascular outcomes. We recommend (1) standardisation of routine collection and recording of cardiometabolic history and risk factors as part of baseline assessment and (2) standardisation of adverse event reporting in accordance with international society guidelines in future studies to better understand the cardiometabolic impact these treatments have on our patients.