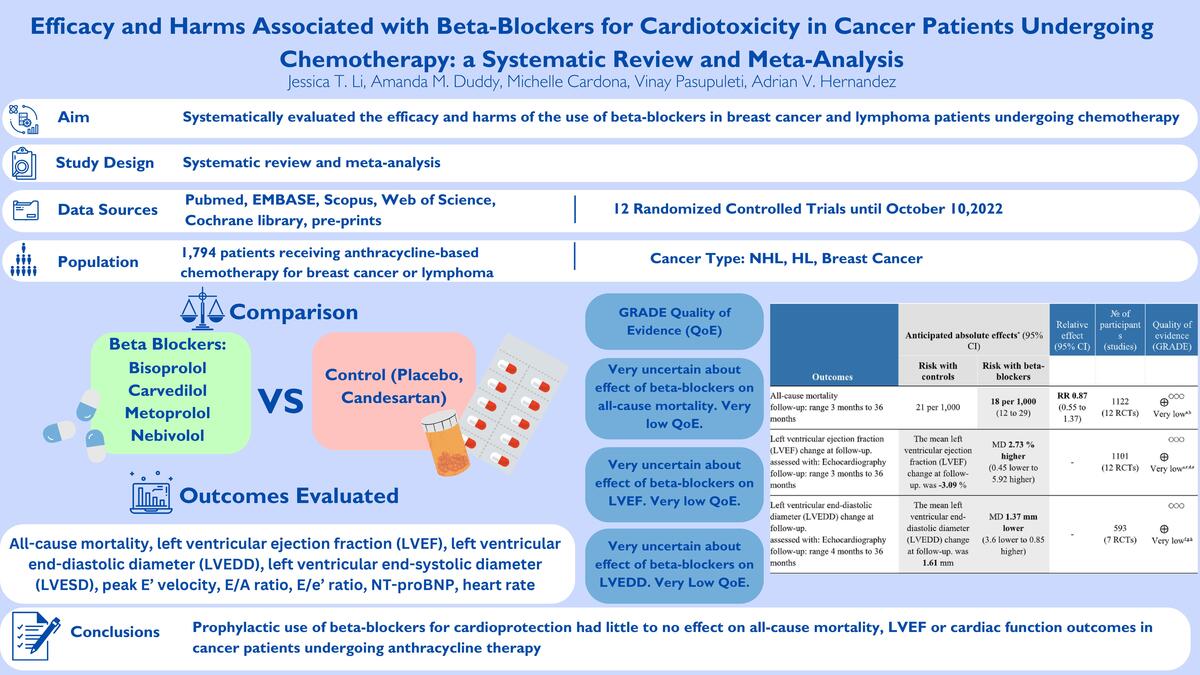
Introduction
Anthracyclines such as doxorubicin, daunorubicin, epirubicin, and idarubicin are the mainstay of treatment for various cancers, including breast cancer and lymphoma [1, 2]. However, they can cause serious complications including left ventricular dysfunction and subsequent heart failure, which has limited their use in cancer patients [3]. Up to 20% of those who have received an anthracycline will develop cardiotoxicity within 5 years [4]. Some risk factors associated with anthracycline-induced cardiotoxicity include cumulative dose, female sex, and pre-existing heart conditions such as arterial hypertension [3]. About 10% of breast cancer patients undergoing anthracycline chemotherapy develop cardiotoxicity, and about 20% of long-term lymphoma survivors treated with anthracyclines have asymptomatic cardiac dysfunction [5–7].
Several strategies have been developed to prevent anthracycline-related cardiotoxicity, including administration alternatives (liposomal, continuous infusion) and the use of cardioprotective drugs according to the 2017 American Society of Clinical Oncology (ASCO) and the 2020 European Society for Medical Oncology (ESMO) guidelines [8–10]. These drugs include dexrazoxane, angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blocker (ARB), and β-blockers [10]. Although dexrazoxane has FDA approval for doxorubicin-related cardiotoxicity, it is more expensive and has an extensive side-effect profile compared to other alternatives such as β-blockers. The proposed mechanisms of benefit of β-blockers include blocking sympathetic activity, optimizing excitation-contraction coupling, and reducing heart rate [11].
The systematic review and meta-analysis by Lewinter et al. in 2022 evaluated seven randomized controlled trials (RCTs) (n = 708) in which β-blocker therapy non-significantly increased left ventricular ejection fraction (LVEF) by 1.9% (95% CI: –0.5% to 4.2%, I2 = 77%) compared to placebo in breast cancer patients receiving anthracyclines only [12]. Also, the systematic review and meta-analysis by Kheiri et al. in 2018 assessed eight RCTs (n = 633) and found that carvedilol significantly increased LVEF by 2.41% (95% CI: 0.01% to 4.81%, I2 = 87%) compared with placebo in cancer patients receiving anthracyclines [13]. Similarly, the meta-analysis by Ma et al. in 2019 evaluating 11 RCTs (n = 940) found a significant increase of LVEF by 4.5% (95% CI: 1.77% to 7.15%), significant reductions in LV end-systolic diameter (LVESD) and LV end-diastolic diameter (LVEDD), and non-significant differences in peak E′ velocity, E/A ratio, and E/e′ ratio with β-blockers compared to placebo in cancer patients receiving anthracyclines [14]. However, none of the previous studies assessed the effect of β-blockers in only breast cancer and lymphoma patients against several comparators, predefined a large set of outcomes, and used state-of-the-art methods to assess the risk of bias (RoB) of individual RCTs and the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) quality of evidence per outcome across RCTs.
We systematically evaluated the efficacy and harms associated with the use of β-blockers for anthracycline-related cardiotoxicity in breast and lymphoma cancer patients undergoing chemotherapy.
Material and methods
We reported our systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [15]. The protocol of our study was registered in the PROSPERO database with modifications (CRD42022368169).
Study searches
We conducted comprehensive literature searches on October 9, 2022, in PubMed, EMBASE, Scopus, Web of Science, Cochrane Library, pre-prints (medrxiv.org, ssrn.com, preprints.com), and ongoing randomized controlled trials (RCTs) at clinicaltrials.gov. There were no time or language limits. The following keywords were used for our search strategy: breast cancer, non-Hodgkin lymphoma (NHL), anthracycline-based chemotherapy, selective β-blockers, non-selective β-blockers, angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB), and RCTs. The full search strategy for PubMed is available in the Supplementary file. The references of included studies were searched for additional records.
Study selection
Three reviewers (JTL, AD, VP) searched engines, pre-print websites, and clinicaltrials.gov to collect records. After removing duplicates in myendnoteweb.com, these were exported to rayyan.ai. Two independent reviewers (JTL, AD) determined the eligibility of the studies by title and abstract content according to inclusion and exclusion criteria. We included: 1) RCTs assessing the effects of β-blockers, either selective (bisoprolol, metoprolol, nebivolol, acebutolol, atenolol) or non-selective (nadolol, labetalol, carvedilol, propranolol, sotalol), with or without ACEI or ARBs vs. controls (placebo, standard of care, ACEI, ARB, combination) for cardiotoxicity in patients ≥ 18 years old; 2) patients receiving anthracycline-based chemotherapy with or without administration of monoclonal antibodies for breast cancer and NHL at a hospital or cancer treatment center; and 3) availability of at least one primary or secondary outcome. Studies were excluded if: 1) patients were undergoing chemotherapy for other types of cancer (e.g. gastric, esophageal), or 2) the study design was not an RCT (cohort, case-control, cross-sectional, systematic review, meta-analysis, conference abstract).
Outcomes
Primary outcomes were all-cause mortality, heart failure, changes at follow-up for left ventricular (LV) diastolic and systolic function (e.g. diastolic dysfunction, peak E′ velocity, E/A ratio, E/e′ ratio, LV volume and diameter, filling pressure, deceleration time, strain rate parameters, LV ejection fraction [LVEF]), NT-proBNP levels, and troponin T levels, and serious adverse events. Secondary outcomes were duration of administration of β-blockers, treatment discontinuation, adverse events (e.g. bradycardia), hypertension, metastatic and nonmetastatic disease.
Data extraction
Data were extracted by two independent reviewers (JTL, AD), and disagreements were resolved by a third reviewer (AVH) if necessary. The extracted data included: 1) year of publication, 2) RCT type, 3) number of participants, 4) country(ies) where the studies were conducted, 5) type of patient (breast cancer, NHL), 6) β-blocker name, dose and duration, 7) comparator dose and duration, 8) time frame of follow-up, 9) median age, 10) sex proportion, 11) stage of cancer, 12) prevalence of comorbidities (i.e. diabetes, hypertension, obesity, coronary artery disease, chronic obstructive pulmonary disease, asthma, chronic kidney disease), 13) primary and secondary outcomes per study arm.
Risk of bias assessment
Assessment of risk of bias (RoB) was done independently by two investigators (JTL, AD) using the Cochrane RoB 2.0 tool for RCTs [16]. This tool evaluates five domains of bias: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Judgements of bias per domain can be “low”, “high”, or “some concerns”. Each study and domain were classified following a predetermined algorithm based on responses to signaling questions. A third reviewer (AVH) participated in the resolution of discrepancies.
GRADE Quality of evidence
The quality of evidence (QoE) was evaluated using the GRADE methodology [17]. The following aspects were assessed per outcome: RoB, inconsistency, imprecision, indirectness, and publication bias. We downgraded the QoE according to limitations per aspect across RCTs to moderate, low, and very low, and provided explanations for each decision. The GRADEpro software (www.gradepro.org) was used to generate the Summary of Findings (SoF) table.
Statistical analysis
All meta-analyses were conducted using a random-effects model with the inverse variance method. The Paule-Mandel method was used to calculate the between-study variance (τ2) [18], and the Hartung-Knapp method was used to adjust 95% confidence intervals (CIs) [19]. Effects were reported as relative risks (RR) and their 95% CIs for dichotomous outcomes, and as mean differences (MD) and their 95%CIs for continuous outcomes. We adjusted for baseline values of continuous outcomes. Zero events in one or two arms were adjusted with the treatment arm continuity correction (TACC) method. Statistical heterogeneity was evaluated using the I2 statistic, with values < 30% meaning low, 30% to 60% moderate, and > 60% high heterogeneity of effects across RCTs [20]. Pre-specified subgroup analyses were conducted by type of patient (breast cancer vs. other population [breast cancer plus lymphoma or lymphoma alone]), type of β-blocker (selective vs. non-selective), type of chemotherapy delivery (hospital vs. cancer treatment center), and by RCT RoB (high vs. low vs. some concerns). We ran sensitivity analyses by excluding RCTs with comparators other than placebo. Small study effects on outcomes were evaluated with funnel plots and Egger’s test when 10 or more RCTs were available. The R 4.2.0 (www.r-project.org) software was used for all meta-analyses.
Results
Study selection
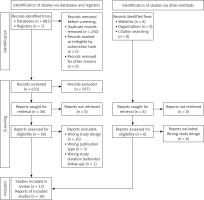
We identified 883 citations from databases and none from registries or pre-print websites (Figure 1). After removing duplicates, we screened 633 by titles and abstract text, and 597 abstracts were excluded. Therefore, 36 studies were assessed for eligibility and 20 studies were excluded because of wrong study design (n = 16), wrong publication type (n = 3), or wrong study duration (n = 1). Finally, 12 unique RCTs were selected for quantitative and qualitative analyses, which were reported in 16 papers [21–36].
Characteristics of included RCTs
Table I shows the main features of the included RCTs [16–31]. Eight of the 12 RCTs assessed solely patients with breast cancer. Four RCTs included other types of cancer such as NHL and lymphoma in the patient population [21, 26, 30, 36]. For the chemotherapy regimen, two RCTs assessed doxorubicin [21, 26], one RCT used doxorubicin and cyclophosphamide [35], three RCTs assessed doxorubicin and cyclophosphamide followed by paclitaxel [20–23], one RCT assessed total doxorubicin dose and total epirubicin dose [30] and one RCT assessed doxorubicin and epirubicin [36]. One RCT assessed cyclophosphamide, epirubicin, and 5FU, or Adriamycin and cyclophosphamide [31], one RCT assessed only epirubicin [27–29], and one RCT assessed either cyclophosphamide and doxorubicin or docetaxel and doxorubicin [32]. One RCT assessed Adriamycin plus cyclophosphamide (AC), fluorouracil plus epirubicin plus cyclophosphamide plus docetaxel (FEC-D), epirubicin plus cyclophosphamide (EC), or fluorouracil plus epirubicin plus cyclophosphamide (FEC-100) for the chemotherapy regimen [33, 34].
Table I
Characteristics of included RCTs
| Author, year, acronym (reference) | Country | Sample size | Cancer type (%) | Chemotherapy | B-blocker dose | Comparator | Age mean, (SD) | Female (%) | Baseline LVEF% Mean ± SD (n) | Follow-up [months] | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kalay, 2006 [30] | Turkey | 50 | BC: 34 (68%) Lymphoma: 9 (18%) | Total doxorubicin dose (mg/m2): carvedilol 525.3, control 513.6; total epirubicin dose (mg/m2): carvedilol 787.9, control 770.4 | Carvedilol 12.5 mg daily, 6 months | Control | 46.8 (14) | 88 | 70.2 ±7.6% (50) | 6 | Primary: Systolic function from baseline to 6 months Secondary: Measured systolic and diastolic diameters of LV (not explicitly stated) |
| Georgakopoulos, 2010 [26] | Greece | 147 | Lymphoma: NHL: 41 (28%); HL: 41 (28%) | Cumulative doxorubicin dose after 8 cycles: metoprolol = 387.5 (6.8); control = 386.4 (5.7); enalapril = 373.1 (6.3) | Metoprolol, 12 months | Control | 51.0 (18.0) | 50 | 66.8 ±6.5% (125) | 36 | Primary: Total cardiotoxicity cases up to 30 months |
| Salehi, 2011 [36] | Iran | 66 | BC: 42 (64%) Lymphoma: 24 (36%) | Doxorubicin 50–60 mg/m2 and epirubicin 100 mg/m2; cumulative doxorubicin dose carvedilol = 521.28 ±31.17 mg/m2; control 540.28 ±31.17 mg/m2 | Carvedilol 25 mg daily, 4 months | Placebo, 1 tab daily, 4 months | 52.52 (11.00) | 46.67 | 66.0 ±5.5% (66) | 4 | Primary: Change in LVEF from baseline to 4 months |
| Kaya, 2012 [31] | Turkey | 45 | BC: 45 (100%) | CEF = cyclophosphamide 500–600 mg/m2 + epirubicin 75–100 mg/m2 + 5FU 500–600 mg/m2; AC = Adriamycin 60 mg/m2 + cyclophosphamide 600 mg/m2 | Nebivolol 5 mg once daily, 6 months | Placebo, 1 tablet daily, 6 months | 50.5 (11.1) | 100 | 66.0 ±5.1% (45) | 6 | Primary: Change in LVEF from baseline to 6 months Secondary: early onset anthracycline induced cardiotoxicity |
| Beheshti, 2016 [24] | Iran | 70 | BC: 70 (100%) | Doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 followed by paclitaxel 175 mg/m2 for 4 cycles (cumulative doxorubicin dose 240 mg/m2) | Carvedilol 6.25 mg BID, 12 weeks | Placebo, BID, 12 weeks | 39.9 | 100 | 60.22 ±3.9% (70) | 10th day after the chemotherapy was finished, 94 days (about 3 months) | Primary: LVEF and strain/strain rates measurement |
| Nabati, 2017 [35] | Iran | 81 | BC: 81 (100%) | Doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks for 6 cycles | Carvedilol 3.125 mg BID, 6 months | Placebo, 1 tab BID, 6 months | 47.57 (8.75) | 100 | 59.9 ±5.0% (81) | 6 | Primary: Change in LVEF before echo and 6 months after randomization in LVEF from baseline to 4 months Secondary: incidence of death, change in LV diastolic dysfunction and LV end diastolic and systolic volumes |
| Abuosa, 2018 [21] | Saudi Arabia | 154 | BC: 72 (47%) Lymphoma: NHL: 36 (23%) | Cumulative doxorubicin dose (mg/m2): carvedilol 25 mg: 261.0 ±101.8; Placebo: 265.6 ±98.5 | Carvedilol 25 mg once a day, 6 months | Placebo, 1 tablet daily, 6 months | 42.0 (15.0) | 77 | 61 ±4.3% (154) | 6 | Primary: The differences in the change in LVEF from baseline to 6 months for patients assigned to placebo compared to carvedilol, regardless of dose Secondary: LVSD, LVDD, E′, E/A, DT, E/E′, mortality, BP, heart rate |
| Avila, 2018; Ayub-Ferreira, 2020; CECCY [22, 23] | São Paulo, Brazil | 192 | BC: 192 (100%) | 4 cycles cyclophosphamide 600 mg/m2 and doxorubicin 60 mg/m2 every 21 days (cumulative dose 240 mg/m2), followed by paclitaxel 80 mg/m2 for 8 weeks | Carvedilol 25 mg BID, 20 weeks | Placebo, BID, 20 weeks | 50.80 (10.10) | 100 | 65 ±4.2% (192) | 6 | Primary: Reduction in LVEF of greater than or equal to 10% at 6 months & 2 years Secondary: troponin I, B-natriuretic peptide, and diastolic dysfunction – Long term: changes in left ventricular diastolic diameter and diastolic dysfunction |
| Cochera, 2018 [25] | Romania | 60 | BC: 60 (100%) | Doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 followed by paclitaxel 175 mg/m2 for 4 cycles (cumulative doxorubicin dose 240 mg/m2) | Nebivolol 5 mg once daily, 18 weeks | Control, 1 tablet daily, 18 weeks | 52.6 (13) | 100 | 61.5 ±3.2% (60) | 6 cycles of chemotherapy (4 months) | Primary: LVEF and strain/strain rate parameters |
| Gulati, 2016; Gulati, 2017; Heck, 2018; PRADA [27–29] | Norway | 120 | BC: 120 (100%) | Epirubicin 100 mg/m2 for 4 cycles or epirubicin 60 mg/m2 for 4 or 6 cycles (cumulative doxorubicin equivalent 268 mg/m2 and 161 mg/m2, respectively) | Metoprolol 100 mg daily, 2 years | No metoprolol, placebo tablet BID, 2 years | 51 (9) | 100 | ** | 23 | Primary: change in LVEF assessed by CV magnetic resonance imaging from baseline, biomarkers troponin I and T, BNP, CRP, and galectin-3 Secondary: Biomarker response and reduction in LVEF |
| Lee, 2021, SAVEHEART [32] | Korea | 152 | BC: 152 (100%) | Either 4 cycles of cyclophosphamide 600 mg/m2 and doxorubicin 60 mg/m2 every 21 days (cumulative dose 60 mg/m2) or 6 cycles of docetaxel 75 mg/m2 and doxorubicin 50 mg/m2 | Carvedilol 3.125 mg daily, 12 months | Candesartan, 4 mg daily, 12 months | 46.6 (7.6) | 100 | 64.8% (IQR 63.0% to 66.2%) (152) | 12 | Primary: Late DISC (at least 12 months after chemotherapy |
| Livi, 2021; Meattini, 2022; SAFE [33, 34] | Italy | 174 | BC (ERBB2-positive): 174 (100%) | AC, FEC-D, EC, FEC-100 | Bisoprolol 5 mg once daily, 24 months | Placebo + placebo, 1 tab morning and 1 tab evening, 24 months | 48.6 (7.9) | 100 | 66.5% (IQR 65.9% to 67.0%) (174) | 24 | Primary: Number of patients with > 10% decrease in LVEF after 12 months Secondary: Evaluation of reduction of both systolic and diastolic, early and late, cardiac damage |
[i] BC – breast cancer, LV – left ventricular, NHL – non-Hodgkin’s lymphoma, HL – Hodgkin’s lymphoma, LVEF – left ventricular ejection fraction, LVSD – left ventricular systolic dysfunction, LVDD – left ventricular diastolic dysfunction, DT – deceleration time, BP – blood pressure, CV – cardiovascular, AC – doxorubicin 60 mg/m2, cyclophosphamide 600 mg/m2 every 21 days for 4 cycles, FEC – epirubicin 100 mg/m2 or 75 mg/m2, cyclophosphamide 500 mg/m2, fluorouracil 500 mg/m2 every 21 days for 3 cycles, followed by docetaxel 100 mg/m2 or 75 mg/m2 every 21 days for 3 cycles, EC – epirubicin 75 mg/m2, cyclophosphamide 600 mg/m2 every 21 days for 4 cycles.
Carvedilol was evaluated in seven of the twelve RCTs; two RCTs assessed carvedilol 3.125 mg, one RCT assessed carvedilol 6.25 mg, one RCT assessed carvedilol 12.5 mg, and three RCTs assessed carvedilol 25 mg [21–24, 30, 32, 35, 36]. Four studies evaluated other β-blockers including bisoprolol, metoprolol, and nebivolol [25–29, 31, 33, 34]. Eleven studies used placebo or unspecified control as a comparator [21–31, 33–36] and one study used candesartan as a comparator [32]. Mean ages ranged from 39.9 to 52.6 years; eight studies included only female participants [21–25, 27–29, 31–35], and the other four studies had female participants ranging from 46.67% to 88% [21, 26, 30, 36]. The follow-up duration ranged from 3 months to 36 months across RCTs. We did not find information on several predefined outcomes, including heart failure, LV volume, filling pressure, deceleration time, serious adverse events, duration of administration of β-blockers, treatment discontinuation, and whether the patient developed metastatic or nonmetastatic disease. LV diastolic dysfunction, strain parameters, troponin T levels, and hypertension were only reported in one RCT [22–24].
Risk of bias assessment
Supplementary Figure S1 displays the RoB assessments of the 12 RCTs, and overall, two were found to have low RoB, nine some concerns of bias, and one high RoB. Three RCTs had some concerns about bias in the randomization process [26–30], six RCTs had some concerns about bias in deviations from intended interventions [21, 25, 30–32, 36], and three RCTs had some concerns about bias in measurement of the outcome [25, 33, 34, 36]. One study had some concerns about bias in selection of the reported result [26], and one study had high risk of bias in selection of the reported result [36].
Effects on primary and secondary outcomes
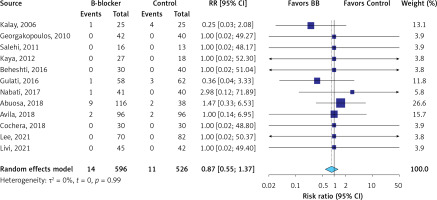
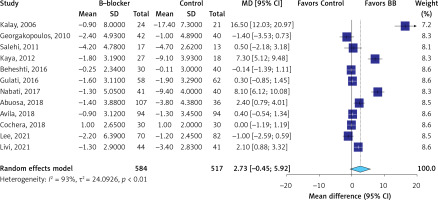
The evidence was very uncertain about the effect of β-blockers on all-cause mortality (RR = 0.87; 95% CI: 0.55 to 1.37, I2 = 0%, 12 RCTs, very low QoE, Figure 2) in comparison to the control group. The evidence was very uncertain about the effect of β-blockers on LVEF (MD = 2.73 %; 95% CI: –0.45 to 5.92, I2 = 93%, 12 RCTs, very low QoE, Figure 3), LVEDD (MD = –1.73 mm; 95% CI: –3.60 to 0.85, I2 = 90%, seven RCTs, very low QoE, Supplementary Figure S2), and LVESD (MD = –1.69 mm; 95% CI: –3.87 to 0.50, I2 = 93%, seven RCTs, very low QoE, Supplementary Figure S3) compared to the control. Also, the evidence was very uncertain about the effect of β-blockers on peak E′ velocity (MD = 6.4. cm/s; 95% CI: –1.71 to 14.52, I2 = 85%, three RCTs, very low QoE, Supplementary Figure S4), E/A ratio (MD = 0.06; 95% CI: –0.01 to 0.14, I2 = 51%, eight RCTs, very low QoE, Supplementary Figure S5), and E/e′ ratio (MD = –0.24; 95% CI: –0.82 to 0.34, I2 = 68%, four RCTs, very low QoE, Supplementary Figure S6) compared to the control group. B-blockers likely reduced NT-pro BNP levels slightly (MD = –15.35 pg/ml, 95% CI: –22.39 to –8.31, two RCTs, I2 = 0%, moderate QoE, Supplementary Figure S7) compared to the control. The evidence was very uncertain about the effect of β-blockers on heart rate (MD = –9.14 bpm, 95% CI: –15.02 to –3.26, I2 = 87%, two studies, very low QoE, Supplementary Figure S8) compared to the control group.
Subgroup and sensitivity analyses
Effects of β-blockers on outcomes across predefined subgroups by type of β-blockers, type of patient population, and RoB are shown in Supplementary Figures S9–S11, respectively. Subgroup analyses were mostly consistent with the main analyses. In subgroup analysis by β-blocker type, we found differential effects on peak E′ velocity with a significant increase with the use of other β-blocker (MD = 14.3 cm/s, 95% CI: 9.57 to 19.03) and not with carvedilol vs. control (p for interaction < 0.01). In subgroup analysis by type of patient population, we found the same differential effects on peak E′ velocity for breast cancer patients, but not in other types of patients, as RCTs were grouped similarly as type of β-blocker (p for interaction < 0.01). In subgroup analysis by patient population, we found differential effects on E/e′ ratio with a significant reduction in breast cancer patients (MD = –0.78, 95% CI: –1.27 to –0.30) and not in other patient populations vs. control (p for interaction < 0.01). Finally, in subgroup analysis by risk of bias, we found differential effects on LVESD with a significant reduction in RCTs at low RoB (MD = –1.00 mm, 95% CI: –1.66 to –0.34) and not in other RCTs (p for interaction < 0.01). We did not assess subgroups by chemotherapy delivery, as this information was not provided by the RCT reports. Sensitivity analyses by excluding RCTs with comparators different from placebo were consistent with main analyses (Supplementary Figure S12).
GRADE quality of evidence per outcome
QoE was very low for most of the primary and secondary outcomes (Table II). QoE was very low for most outcomes due to high RoB, high heterogeneity among effects across RCTs, and imprecision of effect. For NT-proBNP levels, the QoE was moderate due to some concerns of bias across RCTs.
Table II
Summary of findings (SoF) table of the effects of β-blockers on outcomes in patients receiving chemotherapy for breast cancer and lymphoma
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with controls | Risk with β-blockers | ||||
| All-cause mortality Follow-up: range 3 months to 36 months | 21 per 1,000 | 18 per 1,000 (12 to 29) | RR 0.87 (0.55 to 1.37) | 1122 (12 RCTs) | ⨁◯◯◯ Very lowa,b |
| Left ventricular ejection fraction (LVEF) change at follow-up Assessed with: echocardiography Follow-up: range 3 months to 36 months | The mean left ventricular ejection fraction (LVEF) change at follow-up was –3.09 % | MD 2.73% higher (0.45 lower to 5.92 higher) | – | 1101 (12 RCTs) | ⨁◯◯◯ Very lowa,c,d,e |
| Left ventricular end-diastolic diameter (LVEDD) change at follow-up Assessed with: echocardiography Follow-up: range 4 months to 36 months | The mean left ventricular end-diastolic diameter (LVEDD) change at follow-up was 1.61 mm | MD 1.37 mm lower (3.6 lower to 0.85 higher) | – | 593 (7 RCTs) | ⨁◯◯◯ Very lowf,g,h |
| Left ventricular end-systolic diameter (LVESD) change at follow-up Assessed with: echocardiography Follow-up: range 4 months to 36 months | The mean left ventricular end-systolic diameter (LVESD) change at follow-up was 2.11 mm | MD 1.69 mm lower (3.87 lower to 0.5 higher) | – | 593 (7 RCTs) | ⨁◯◯◯ Very lowc,f,i |
| Peak E′ velocity change at follow-up Assessed with: echocardiography Follow-up: range 4 months to 6 months | The mean peak E velocity change at follow-up was –9.1 cm/s | MD 6.41 cm/s higher (1.71 lower to 14.52 higher) | – | 120 (3 RCTs) | ⨁◯◯◯ Very lowj,k,l |
| E/A ratio change at follow-up Assessed with: echocardiography Follow-up: range 4 months to 36 months | The mean E/A ratio change at follow-up was –0.097 | MD 0.06 higher (0.01 lower to 0.14 higher) | – | 571 (8 RCTs) | ⨁◯◯◯ Very lowm,n,o |
| E/e’ ratio change at follow-up Assessed with: echocardiography Follow-up: range 4 months to 36 months | The mean e/e’ ratio change at follow-up was 0.36 | MD 0.24 lower (0.82 lower to 0.34 higher) | - | 330 (4 RCTs) | ⨁◯◯◯ Very lowp,q,r |
| NT-proBNP level change at follow-up Assessed with: plasma sample Follow-up: range 6 months to 23 months | The mean NT-proBNP level change at follow-up was 26.7 pg/ml | MD 15.35 pg/ml lower (22.39 lower to 8.31 lower) | - | 165 (2 RCTs) | ⨁⨁⨁◯ Moderates |
| Heart rate change at follow-up Assessed with: clinical assessment Follow-up: range 6 months to 24 months | The mean heart rate change at follow-up was –0.59 bpm | MD 9.14 bpm lower (15.02 lower to 3.26 lower) | - | 130 (2 RCTs) | ⨁◯◯◯ Very lowt,u |
a RoB: Downgraded two levels because one RCT was at high risk of bias, and nine RCTs were at some concerns of bias.
b Imprecision: Downgraded one level as 95% CI is from 0.55 to 1.37, and limits have opposite direction.
d Imprecision: Downgraded one level as 95%CI is from –0.45% to 5.92%, and limits have opposite direction.
f RoB: Downgraded two levels because one RCT was at high risk of bias, and five RCTs were at some concerns of bias.
h Imprecision: Downgraded one level as 95% CI is from –3.6 mm to 0.85 mm, and limits have opposite direction.
i Imprecision: Downgraded one level as 95% CI is from –3.87 mm to 0.5 mm, and limits have opposite direction.
j RoB: Downgraded two levels because 1 RCT has high risk of bias and 2 RCTs were at some concerns of bias.
l Imprecision: Downgraded two levels as 95% CI is from –1.71 to 14.52, and limits have opposite direction.
m RoB: Downgraded two levels because one RCT was at high risk of bias, and 7 RCTs were at some concerns of bias.
o Imprecision: Downgraded one level as 95% CI is from –0.01 to 0.14, and limits have opposite direction.
r Imprecision: Downgraded one level as 95% CI is from –0.82 to 0.34, and limits have opposite direction.
Discussion
Main findings
In our systematic review of RCTs, we found that β-blockers did not improve most clinical and intermediate outcomes related to cardiotoxicity in patients with breast cancer and lymphoma. There were non-significant reductions in all-cause mortality risk, and non-significant improvements in other intermediate outcomes of cardiac function (LVEF, LVESD, LVEDD, E/e′ ratio) vs. controls. Also, we found significant reductions in NT-proBNP levels and heart rate with the use of β-blockers vs. controls. Subgroup analyses by type of β-blocker, by cancer patient population, and by RoB were mostly consistent with main analyses. However, the GRADE QoE of primary and secondary outcomes was very low, except for NT-proBNP levels, which had moderate QoE.
What is known about our research question in the literature
In 2022, a systematic review and meta-analysis by Lewinter et al. evaluated nine RCTs (n = 1,362) up to March 2021 that solely focused on patients with breast cancer, and assessed effects of β-blockers, ARBs and ACEI in patients receiving anthracyclines or trastuzumab. Seven RCTs (n = 708) focused on effects of β-blockers in patients receiving anthracyclines. B-blockers included bisoprolol, carvedilol, metoprolol, and nebivolol, and the authors found that β-blocker therapy non-significantly increased LVEF by 1.9% compared to placebo in breast cancer patients (95% CI: –0.5% to 4.2%, I2 = 77%) [12]. This meta-analysis only evaluated LVEF as an outcome without adjustment for baseline values and was somewhat limited in its research sources due to only using PubMed, EMBASE and CENTRAL. They used the DerSimonian-Laird method to calculate between-study variance τ2 instead of more recommended methods such as the Paule-Mandel, empirical Bayes or Sidik-Jonkman methods. Additionally, the authors did not evaluate the QoE per outcome.
The systematic review and meta-analysis by Kheiri et al. in 2018 evaluated eight RCTs (n = 633) up to March 2018. The patient population included those with breast cancer, lymphoreticular malignancy, lymphoma, and various malignancies. The authors found that carvedilol significantly increased LVEF by 2.41% (95% CI: 0.01% to 4.81%, I2 = 87%) and significantly reduced the odds of having low EF (i.e. LVEF < 50%) by 48% (odds ratio [OR] = 0.42, 95% CI: 0.18 to 0.99) compared to placebo in cancer patients [13]. Some limitations of this meta-analysis included only looking at LVEF as an outcome, not pre-specifying low EF in their protocol, and not assessing effects on the protocol pre-specified outcomes heart failure and myocardial infarction. Also, the authors only focused on carvedilol as the intervention, not specifying the carvedilol doses that were used. Finally, the authors used the outdated Jadad score to assess risk of bias of RCTs and the wrong PRISMA guidelines to report their systematic review and did not evaluate the QoE.
In 2019, a systematic review and meta-analysis by Ma et al. evaluated 11 RCTs (n = 940). The patient population included those with lymphoma, breast cancer, lymphoreticular malignancy, acute leukemia, and unspecified patients with malignancy. The interventions included carvedilol 5 mg twice daily, carvedilol 25 mg twice daily, metoprolol 100 mg daily, and candesartan 32 mg with metoprolol 100 mg daily. The authors found that β-blockers led to a significant reduction in symptomatic heart failure (RR = 0.29, 95% CI: 0.10 to 0.85), a significant increase of LVEF by 4.5% (95% CI: 1.77% to 7.15%), significant reductions in LVESD by 3.19 mm (95% CI: –6.17 to –0.21 mm), and significant reduction in LVEDD by 2.28 mm (95% CI: –4.50 mm to –0.05 mm) compared to placebo [14]. There were no significant differences in peak E′ velocity, E/A ratio, and E/e′ ratio with β-blockers compared to placebo. Some limitations of this meta-analysis included not having a registered protocol in PROSPERO, not specifying the dates of searches, using PRISMA guidelines to conduct a systematic review, and not assessing QoE. Finally, the systematic review and meta-analysis by Xu et al. in 2020 evaluated 11 RCTs (n = 844) until January 2019, which included patients with breast cancer, lymphoma, NHL, and HL. Interventions included were carvedilol 12.5 mg/day and nebivolol 5 mg/day. They found a significant increase in LVEF by 2.87% (95% CI: 0.64% to 5.11%) [37]. Limitations of this meta-analysis included only looking at LVEF as an outcome and not evaluating the QoE.
The 2017 ASCO guidelines recommended that clinicians may incorporate the use of dexrazoxane, continuous infusion or liposomal formulation of doxorubicin for prevention of cardiotoxicity in patients planning on receiving high-dose anthracyclines (strength of recommendation: moderate; evidence quality: intermediate) [9]. In 2020, the ESMO guidelines recommended the prophylactic use of ACEI or ARBs, and/or selected β-blockers such as carvedilol and nebivolol may be considered to reduce the development of cardiotoxicity for patients undergoing anticancer therapy with known cardiotoxic agents (class of recommendation [COR] III, level of evidence (LOE) C) [10]. The 2022 AHA/ACC heart failure guidelines recommended that in patients at risk of cancer therapy-related cardiomyopathy, the initiation of β-blockers and ACEI/ARB for the primary prevention of drug-induced cardiomyopathy is of uncertain benefit (COR 2b, LOE B-R) [38]. Finally, the 2021 European Society of Cardiology guidelines recommended that treatment with an ACEI and a β-blocker (preferably carvedilol) should be considered in cancer patients developing LV systolic dysfunction, defined as a 10% or more decrease in LVEF and to a value lower than 50% during anthracycline chemotherapy (COR 11a, LOE B) [39].
What our work adds to the literature
Our study had some strengths. First, we used numerous engines, websites, and pre-prints to conduct a comprehensive literature search until October 2022, which is more recent than other published meta-analyses. Second, we used the updated RoB2 tool for assessment of RoB. Third, we used several predefined outcomes that were available in our protocol, and there was no restriction on the comparators; however, we did not find data on several of those outcomes. Fourth, we conducted prespecified subgroup analyses and sensitivity analyses, and we reported them as secondary findings. Finally, we used the GRADE methodology to assess QoE per outcome, and we provided a description of effects not only based on significance but also on risk of bias across RCTs, imprecision of the effects, the degree of heterogeneity of effects among RCTs and the probability of publication bias.
Our meta-analysis showed that β-blockers can increase LVEF, peak E′ velocity, and E/A ratio, and lower all-cause mortality risk, E/e′, LVESD, LVEDD, NT-proBNP, and heart rate in comparison to controls. According to known β-blocker effects, most of our findings were expected except for peak E′ velocity and E/A ratio. Normally, β-blockers would decrease both peak E′ velocity and E/A ratio; reasons for this discrepancy may involve having few RCTs or very low QoE for such outcomes. The meta-analysis by Ma et al. in 2019 found effects with the same direction as ours, but significant effects for LVEF, LVEDD, and LVESD vs. placebo [14]. These discrepancies may be related to the inclusion of a different set of RCTs, which controlled for placebo only, with a broader patient population (e.g. including acute leukemia, malignant hemopathies receiving stem cell transplantation or lymphoreticular malignancy) [40, 41], and with a combined intervention (i.e. carvedilol plus candesartan) [42]. Ma et al. also included an RCT with a misleading abstract describing a case report and no effects on outcomes [43].
Limitations
Our study has several limitations. First, the data derived from published RCTs and their supplementary materials; there was no contact with the authors of those RCTs for extra outcome information. Second, there were only a few events for all-cause mortality across RCTs; we adjusted our analyses for the presence of rare outcomes. Third, most of the effects of β-blockers on outcomes had very low QoE, which means that more RCTs are needed to conclusively determine the efficacy and safety of β-blockers. Fourth, we did not evaluate individual β-blockers due to scarcity of RCTs for most of those β-blockers. Most of the RCTs used carvedilol for the intervention, which was also seen in the network meta-analysis in 2022 by He et al. [44]. Finally, there was no comparison with other drug classes such as dexrazoxane, which is FDA approved for doxorubicin-induced cardiotoxicity. A meta-analysis in 2019 by Macedo et al. found that dexrazoxane reduced the risk of clinical heart failure (RR = 0.19, 95% CI: 0.09 to 0.40) and cardiac events in patients with breast cancer undergoing anthracycline chemotherapy (RR = 0.36, 95% CI: 0.27 to 0.49) [45].
Conclusions
Our meta-analysis showed that β-blocker therapy did not improve most cardiac function outcomes related to anthracycline-related cardiotoxicity in breast cancer and lymphoma patients. The lack of high-quality evidence precludes the recommendation of using β-blockers in cancer patients undergoing chemotherapy. Additional RCTs are still needed to reach a definite conclusion about the effects on these outcomes, as the existing evidence, except for NT-proBNP levels, had very low QoE. Future RCTs should be performed including β-blockers other than carvedilol, and addressing additional important outcomes such as quality of life.