Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ORTHOPEDICS AND TRAUMATOLOGY / CLINICAL RESEARCH
Causal relationship between 91 circulating inflammatory proteins and osteomyelitis risk: evidence from a Mendelian randomization study
1
Shanxi Medical University, Shanxi, China
2
The Second Hospital of Shanxi Medical University, Shanxi, China
3
The Fourth People’s Hospital of Taiyuan, Shanxi, China
Submission date: 2024-12-16
Final revision date: 2025-04-01
Acceptance date: 2025-04-19
Online publication date: 2025-06-08
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Recent research on bone immunology highlights the role of circulating inflammatory proteins in the progression of osteomyelitis (OM). We aimed to investigate the causal relationship between circulating inflammatory protein levels and OM.
Material and methods:
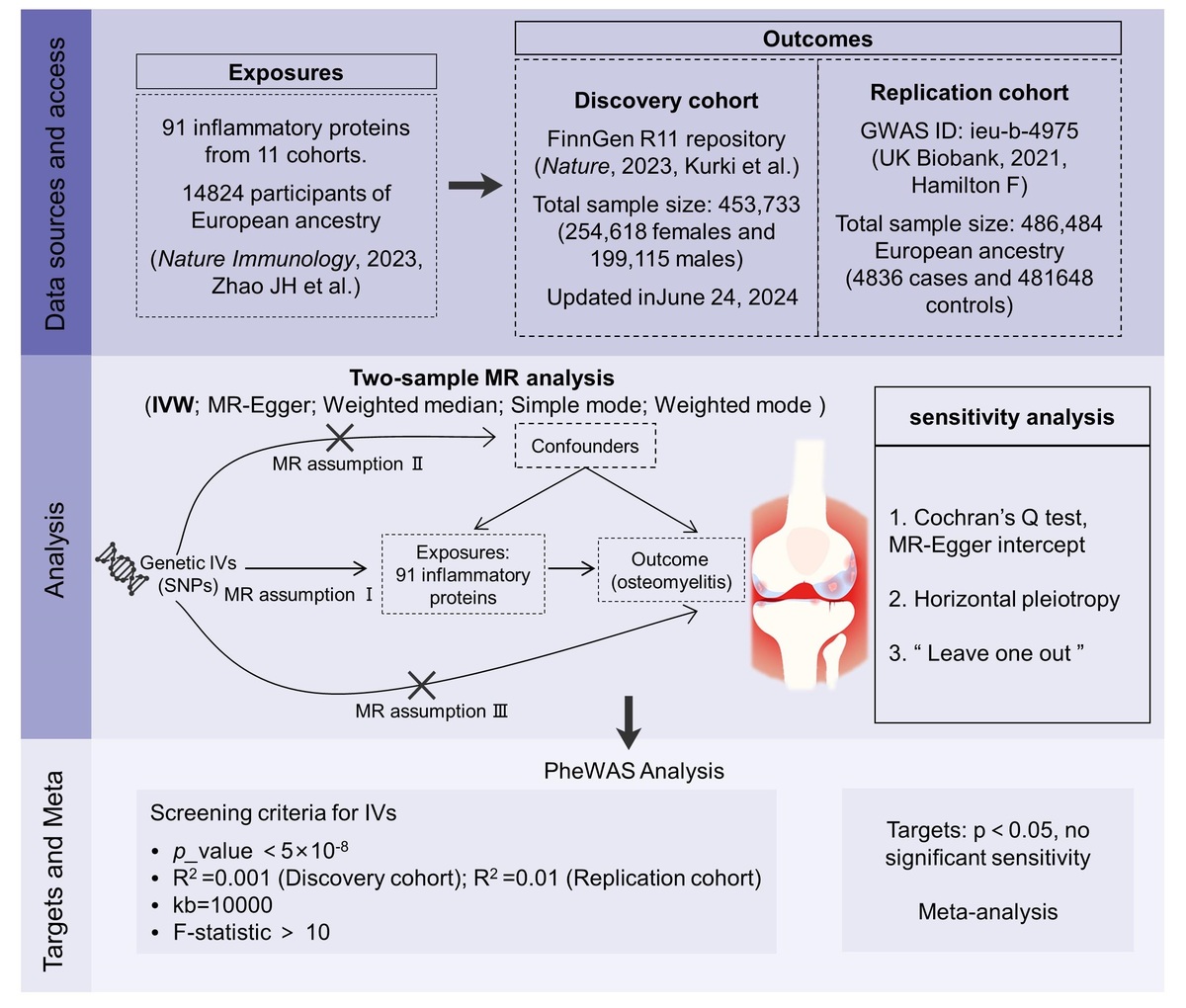
We used summary statistics of 91 inflammatory cytokines (n = 14,824) to perform Mendelian randomization (MR) with two different cohorts of OM. In the discovery phase, summary statistics of OM were obtained from the FinnGen R11 database (2,125 cases vs. 429,826 controls), and the results were replicated in a larger cohort of OM from the UK Biobank study (4,836 cases vs. 481,648 controls). The results of the two MR analyses were applied to a random effects model for meta-analysis. The inverse variance-weighted (IVW) method was used as the main method for MR analysis. We conducted a series of sensitivity analyses to confirm the stability of the causal effect, and used phenotype-wide association analysis (PheWAS) to examine the potential pleiotropy in the study.
Results:
Genetic evidence suggests a causal association between CCL4 (ORIVW = 1.11, 95% CI = 1.04–1.19) and OM in different European ancestry meta-analysis results, which was confirmed by robustness in sensitivity tests and PheWAS. Additionally, osteoprotegerin, MCP-4, ADA, IL15RA, and artemin were positively associated with the risk of OM, while MCP-3, C-X-C motif chemokine 5, and C-C motif chemokine 19 were negatively associated.
Conclusions:
Our findings suggest a potential causal association between 9 inflammatory proteins and OM. However, this study is based on a European ancestry cohort. Future studies are needed to validate these associations in multi-ethnic cohorts and elucidate the biological mechanisms through experimental studies.
Recent research on bone immunology highlights the role of circulating inflammatory proteins in the progression of osteomyelitis (OM). We aimed to investigate the causal relationship between circulating inflammatory protein levels and OM.
Material and methods:
We used summary statistics of 91 inflammatory cytokines (n = 14,824) to perform Mendelian randomization (MR) with two different cohorts of OM. In the discovery phase, summary statistics of OM were obtained from the FinnGen R11 database (2,125 cases vs. 429,826 controls), and the results were replicated in a larger cohort of OM from the UK Biobank study (4,836 cases vs. 481,648 controls). The results of the two MR analyses were applied to a random effects model for meta-analysis. The inverse variance-weighted (IVW) method was used as the main method for MR analysis. We conducted a series of sensitivity analyses to confirm the stability of the causal effect, and used phenotype-wide association analysis (PheWAS) to examine the potential pleiotropy in the study.
Results:
Genetic evidence suggests a causal association between CCL4 (ORIVW = 1.11, 95% CI = 1.04–1.19) and OM in different European ancestry meta-analysis results, which was confirmed by robustness in sensitivity tests and PheWAS. Additionally, osteoprotegerin, MCP-4, ADA, IL15RA, and artemin were positively associated with the risk of OM, while MCP-3, C-X-C motif chemokine 5, and C-C motif chemokine 19 were negatively associated.
Conclusions:
Our findings suggest a potential causal association between 9 inflammatory proteins and OM. However, this study is based on a European ancestry cohort. Future studies are needed to validate these associations in multi-ethnic cohorts and elucidate the biological mechanisms through experimental studies.
REFERENCES (43)
2.
Bhattacharya R, Kundu B, Nandi SK, Basu D. Systematic approach to treat chronic osteomyelitis through localized drug delivery system: bench to bed side. Mater Sci Eng C Mater Biol Appl 2013; 33: 3986-93.
3.
Morgenstern M, Kühl R, Eckardt H, et al. Diagnostic challenges and future perspectives in fracture-related infection. Injury 2018; 49 Suppl 1: S83-90.
4.
Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis 2012; 54: 393-407.
5.
Kremers HM, Nwojo ME, Ransom JE, Wood-Wentz CM, Melton LJ 3rd, Huddleston PM 3rd. Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J Bone Joint Surg Am 2015; 97: 837-45.
6.
Klosterhalfen B, Peters KM, Tons C, Hauptmann S, Klein CL, Kirkpatrick CJ. Local and systemic inflammatory mediator release in patients with acute and chronic posttraumatic osteomyelitis. J Trauma 1996; 40: 372-8.
7.
Massaccesi L, Galliera E, Pellegrini A, Banfi G, Corsi Romanelli MM. Osteomyelitis, oxidative stress and related biomarkers. Antioxidants 2022; 11: 1061.
8.
Asadi A, Razavi S, Talebi M, Gholami M. A review on anti-adhesion therapies of bacterial diseases. Infection 2019; 47: 13-23.
9.
Liesenborghs L, Meyers S, Lox M, et al. Staphylococcus aureus endocarditis: distinct mechanisms of bacterial adhesion to damaged and inflamed heart valves. Eur Heart J 2019; 40: 3248-59.
10.
Ponzetti M, Rucci N. Updates on osteoimmunology: what’s new on the cross-talk between bone and immune system. Front Endocrinol (Lausanne) 2019; 10: 236.
11.
Dapunt U, Maurer S, Giese T, Gaida MM, Hänsch GM. The macrophage inflammatory proteins MIP1 (CCL3) and MIP2 (CXCL2) in implant-associated osteomyelitis: linking inflammation to bone degradation. Mediators Inflamm 2014; 2014: 728619.
12.
Yu X, Huang Y, Collin-Osdoby P, Osdoby P. CCR1 chemokines promote the chemotactic recruitment, RANKL development, and motility of osteoclasts and are induced by inflammatory cytokines in osteoblasts. J Bone Miner Res 2004; 19: 2065-77.
13.
Jordan LA, Erlandsson MC, Fenner BF, et al. Inhibition of CCL3 abrogated precursor cell fusion and bone erosions in human osteoclast cultures and murine collagen-induced arthritis. Rheumatology 2018; 57: 2042-52.
14.
Hemmatzadeh M, Ahangar Parvin E, Mohammadi H, Azizi G, Shomali N, Jadidi-Niaragh F. The role of immune regulatory molecules in rheumatoid arthritis: implication for etiopathogenesis and prospective for treatment. J Cell Physiol 2022; 237: 3541-53.
15.
Cai XP, Zhao Q, Guo ZD, et al. Potential diagnostic value of PD-1 in peripheral blood mononuclear cells of postmenopausal osteoporosis patients. J Clin Lab Anal 2020; 34: e23223.
16.
Larsson SC, Butterworth AS, Burgess S. Mendelian randomization for cardiovascular diseases: principles and applications. Eur Heart J 2023; 44: 4913-24.
17.
Sekula P, Del Greco M F, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol 2016; 27: 3253-65.
18.
Zhao JH, Stacey D, Eriksson N, et al. Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat Immunol 2023; 24: 1960.
19.
Liang YC, Jia MJ, Li L, Liu DL, Chu SF, Li HL. Association of circulating inflammatory proteins with type 2 diabetes mellitus and its complications: a bidirectional Mendelian randomization study. Front Endocrinol 2024; 15: 1358311.
20.
Kurki MI, Karjalainen J, Palta P, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023; 613: 508-18.
21.
Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015; 12: e1001779.
22.
Fan G, Lin L, Xu C. Causal association between body mass index and dilated cardiomyopathy: a Mendelian randomization study. Arch Med Sci 2024; 20: 2040-2.
23.
Zhang B, Zhang R, Ren H, Guan Q, Fan W, Han L. Mendelian randomization analysis of the causal relationship between trimethylamine N-oxide and its precursors and Parkinson’s disease. Arch Med Sci 2024; 20: 1985-92.
24.
Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol 2011; 40: 740-52.
25.
Burgess S, Davey Smith G, Davies NM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res 2023; 4: 186.
26.
Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018; 362: k601.
27.
Wei D, Jiang Y, Cheng J, Wang H, Sha K, Zhao J. Assessing the association of leukocyte telomere length with ankylosing spondylitis and rheumatoid arthritis: a bidirectional Mendelian randomization study. Front Immunol 2023; 14: 1023991.
28.
Wang Q, Dhindsa RS, Carss K, et al. Rare variant contribution to human disease in 281,104 UK Biobank exomes. Nature 2021; 597: 527-32.
29.
Hoy DG, Smith E, Cross M, et al. Reflecting on the global burden of musculoskeletal conditions: lessons learnt from the global burden of disease 2010 study and the next steps forward. Ann Rheum Dis 2015; 74: 4-7.
30.
Luo H, Bauer A, Nano J, et al. Associations of plasma proteomics with type 2 diabetes and related traits: results from the longitudinal KORA S4/F4/FF4 Study. Diabetologia 2023; 66: 1655-68.
31.
Sun Q, Cai D, Liu D, et al. BCL6 promotes a stem-like CD8+ T cell program in cancer via antagonizing BLIMP1. Sci Immunol 2023; 8: eadh1306.
32.
Festa BP, Siddiqi FH, Jimenez-Sanchez M, et al. Microglial-to-neuronal CCR5 signaling regulates autophagy in neurodegeneration. Neuron 2023; 111: 2021-37.
33.
Littlewood-Evans AJ, Hattenberger MR, Lüscher C, Pataki A, Zak O, O’Reilly T. Local expression of tumor necrosis factor alpha in an experimental model of acute osteomyelitis in rats. Infect Immun 1997; 65: 3438-43.
34.
Marriott I, Hughes FM Jr, Bost KL. Bacterial infection of osteoblasts induces interleukin-1beta and interleukin-18 transcription but not protein synthesis. J Interferon Cytokine Res 2002; 22: 1049-55.
35.
Bost KL, Ramp WK, Nicholson NC, Bento JL, Marriott I, Hudson MC. Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of interleukin-6 and interleukin-12 production. J Infect Dis 1999; 180: 1912-20.
36.
Horst SA, Hoerr V, Beineke A, et al. A novel mouse model of Staphylococcus aureus chronic osteomyelitis that closely mimics the human infection: an integrated view of disease pathogenesis. Am J Pathol 2012; 181: 1206-14.
37.
Zheng Q, Wang D, Lin R, et al. Effects of circulating inflammatory proteins on osteoporosis and fractures: evidence from genetic correlation and Mendelian randomization study. Front Endocrinol 2024; 15: 1386556.
38.
Ning R, Zhang X, Guo X, Li Q. Staphylococcus aureus regulates secretion of interleukin-6 and monocyte chemoattractant protein-1 through activation of nuclear factor kappaB signaling pathway in human osteoblasts. Braz J Infect Dis 2011; 15: 189-94.
39.
Söderström K, Stein E, Colmenero P, et al. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc Natl Acad Sci USA 2010; 107: 13028-33.
40.
Feng S, Madsen SH, Viller NN, et al. Interleukin-15-activated natural killer cells kill autologous osteoclasts via LFA-1, DNAM-1 and TRAIL, and inhibit osteoclast-mediated bone erosion in vitro. Immunology 2015; 145: 367-79.
41.
Votta BJ, White JR, Dodds RA, et al. CKbeta-8 [CCL23], a novel CC chemokine, is chemotactic for human osteoclast precursors and is expressed in bone tissues. J Cell Physiol 2000; 183: 196-207.
42.
Nencini S, Ringuet M, Kim DH, et al. GDNF, Neurturin, and artemin activate and sensitize bone afferent neurons and contribute to inflammatory bone pain. J Neurosci 2018; 38: 4899-911.
43.
Tong Q, Wang XL, Li SB, et al. Combined detection of IL-6 and IL-8 is beneficial to the diagnosis of early stage esophageal squamous cell cancer: a preliminary study based on the screening of serum markers using protein chips. Onco Targets Ther 2018; 11: 5777-87.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.