Introduction
Cardiovascular disease (CVD) accounts for an enormous burden of morbidity and mortality throughout the world. Classic CVD risk factors contributing to the development of atherosclerotic CVD (ASCVD) include hypertension, diabetes mellitus, dyslipidaemia, smoking, and obesity. The presence of these risk factors leads to endothelial dysfunction and oxidative stress, which contribute to the pathophysiological processes of atherosclerosis [1–4]. Between 1962 and 2015, there were over a dozen epidemiological studies conducted in Poland, which concerned cardiovascular risk factors, and except for the LIPIDOGRAM studies, they did not relate specifically to patients attending primary health care (PHC).
The previous LIPIDOGRAM cohort studies (2004, 2006, 2010) showed the distribution of cardiovascular risk factors throughout 6 years in an adult population representative of patients attending PHC in Poland, as well as the efficacy of hypercholesterolaemia treatment with more than 2-year follow-up, and a series of studies on obesity in relation to other cardiovascular risk factors and coronary artery diseases (CAD) [5–7]. The current edition of the LIPIDOGRAM study updates the data on cardiovascular risk factors in the PHC setting, for the first time investigates the prevalence of familial hypercholesterolaemia, gives more details on the therapy, and gives an insight into the pathophysiology of coronary artery disease by analysing oxidative stress, autoantibodies, and gene variants conferring susceptibility to ASCVD.
Oxidative stress is the result of an imbalance between reactive oxygen species (ROS) production and utilisation. ROS are reactive intermediates of molecular oxygen. Under physiological conditions, they are formed in cells as byproducts of cellular metabolism and act as important second messengers that transduce intracellular signals involved in various biological process. Many factors can induce ROS generation, such as ultraviolet radiation, smoking, alcohol consumption, exposure to pollutants, or microbial infection resulting in a respiratory burst. When overproduction of ROS exceeds the buffering capacity of the antioxidant defence systems or when antioxidants are defective, oxidative stress occurs, resulting in oxidative damage to lipids, deoxyribonucleic acid (DNA), and carbohydrates [3]. Increased levels of ROS have been reported in several diseases, including atherosclerosis, CVD, neurodegenerative disorders, cancer, renal diseases, pulmonary diseases, and cardiometabolic diseases [8].
Excessive production of ROS and the insufficiency of enzymatic and non-enzymatic defence mechanisms lead to the oxidation of low-density lipoprotein (LDL) and a decrease in the bioavailability of nitric oxide (NO) in endothelial cells, which begin to release the pro-inflammatory cytokines and express adhesion molecules, such as tumour necrosis factor α (TNF-α), interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and intercellular adhesion molecule-1 (ICAM-1). These mediators promote adhesion and infiltration of monocytes, production of foam cells, growth of smooth muscle or endothelial cells, and increased activity of matrix metalloproteinases. All of these pathophysiological processes potentiate endothelial dysfunction and atheromatous plaque formation in conjunction with impaired lipid metabolism. On the other hand, pro-inflammatory cytokines may lead to increased release of ROS maintaining a positive feedback loop. Additionally, the accumulation of oxidative protein and lipid damage may induce the production of antibodies directed against altered proteins of the body [9–15].
Autoimmune diseases (AD) are disorders in which the immune response to self-antigens results in damage or dysfunction of tissues with a very diverse clinical picture and complicated pathogenetic mechanisms. AD can be systemic or can affect specific organs, cell types, and body systems [16]. Thus far, the aetiology of autoantibody (AAb) formation has not been fully understood. There are many suspected factors that increase the risk of AAb production, including genetic predisposition and environmental factors such as infections, oxidative stress factors, physical and chemical agents, as well as stressful life events [1, 17–20]. In addition to well-known AAb, such as antinuclear antibodies (ANA) or anti-cyclic citrullinated peptides (anti-CCP), some lesser-investigated AAb, such as antibodies against citrullinated forms of enolase (CEP-1α) and dense fine speckled 70 antigen (DSF70), may also be associated with cardiometabolic diseases [18]. On the other hand, increased mortality due to CVD in patients with recognised rheumatoid arthritis and systemic lupus erythematosus confirms that inflammation and oxidative stress promote their development [8, 9, 21]. The role of inflammation in the pathogenesis of atherosclerosis and diabetes mellitus is well documented [22]. In light of the above, it is postulated that the parameters characterising both processes may serve as biomarkers of cardiovascular risk. In this context, the role of C-reactive protein (CRP, or high-sensitivity CRP (hsCRP)) or certain proinflammatory cytokines is well documented [23]. However, the potential significance of markers associated with oxidative stress (especially enzymatic), antinuclear antibodies (ANA), and anti-citrullinated protein/peptide antibodies (ACPA) have not yet been determined. Despite the wide body of evidence implicating inflammation, oxidative stress, and the activity of ROS in animal models of CVD, the exact mechanisms involved in the pathogenesis of CVD are still not fully understood.
CVD, stroke, diabetes mellitus, and metabolic syndrome are a result of complex interplay between genetic and environmental factors. On one hand, detrimental dietary habits may run in families [24], and on the other hand, there is a clear genetic susceptibility to obesity and different cardiometabolic diseases [25–27]. Polygenic risk scores for developing CVD and diabetes are extensively studied with promising results [28, 29], although only by analysing large quantities of data from different populations can such scores be properly validated.
Based on the abovementioned, the main aim of The LIPIDOGRAM2015 and LIPIDOGEN2015 study – a nationwide study of cardiovascular health in primary care in Poland – was to estimate the prevalence of risk factors of atherosclerotic cardiovascular disease, frequency of cardiovascular and related disorders, and their treatment in the primary care setting of Poland. Additionally, a sub-study, LIPIDOGEN2015, was planned, which comprised up to 15% of randomly selected recruited patients and was designed to analyse parameters related to lipid metabolism, atherosclerosis, oxidative stress, inflammatory responses, autoimmune disorders, and gene variants that confer susceptibility to CVD and cardiometabolic diseases.
Material and methods
Design
A nationwide cross-sectional study, LIPIDOGRAM2015, was carried out in Poland in the fourth quarter of 2015 and the first and second quarters of 2016.
Setting
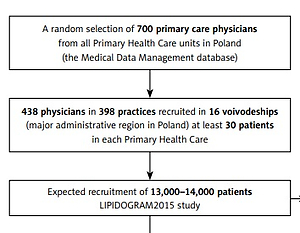
A group of 700 physician-investigators working in public or private primary health care practices were randomly selected by the principal investigators from the Medical Data Management database using computer software. The group of 700 physician-investigators was selected in a manner proportional to the number of inhabitants in a given administrative region (a so-called voivodeship). It was assumed that each physician-investigator would include at least 30 patients under his/her medical care in the study. To reach the required sample size representative for all administrative regions in Poland, the number of patients included in the survey in the smallest Polish voivodeship (Lubuskie voivodeship – 2.64% of the population of Poland) should not be lower than 550 people. Finally, a total of 438 physicians in 398 practices in 16 major administrative regions were recruited.
Participants
Between October 2015 and June 2016, a total of selected primary care physicians actively enrolled consecutive primary care patients (age ≥ 18 years, who were under the care of a physician-investigator during the recruitment period) who sought medical care for any medical reason in primary health care practices. All patients were of Caucasian Polish ethnicity. Prior to data collection, each physician-investigator undertook individual training related to the study procedure and methodology as described in the LIPIDOGRAM2015 and LIPIDOGEN2015 Protocol. The main exclusion criteria were dementia/mental disease resulting in an inability to provide informed consent or lack of consent to participate in the study (Table I).
Table I
Eligibility criteria for the LIPIDOGRAM2015 and LIPIDOGEN2015 studies
Measurements
For each patient recruited for the study, a 28-item questionnaire gathered data on chronic diseases and their treatment, lifestyle (diet, physical activity, smoking status), and family history of CVD (altogether 24 questions). The questionnaire also recorded demographic data: age, sex, place of residence, and level of education (altogether four questions). The questionnaire was first validated in a group of 10 primary care physicians who did not submit comments or noted difficulties in completing it. The content validity was checked by comparing the questionnaire with other similar tools used in Poland (the English translation of the questionnaire is shown in Appendix S1). Each questionnaire was labelled with an individual barcode, identical to barcodes on samples of blood and saliva. A nine-digit code was used – the first digit indicated the edition of the LIPIDOGRAM study (in this case the number 3 indicates year 2015), the next two digits were identical to the code of the district accordingly to National Health Fund (01-16), the next two digits were identical to the code of the physician-investigator in a given district (0-99), and the last four digits indicated the patient number for a given physician-investigator (0001-0030). Data from the paper version of the questionnaire was scanned using a handwriting recognition program. Data of biochemical assays was electronically transferred directly from a biochemical analyser to the appropriate electronic records of the study participant using the nine-digit code mentioned above.
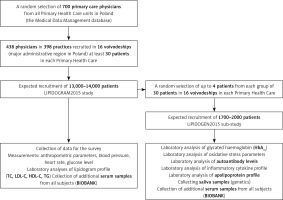
For all patients, anthropometric measurements were made (height, body weight, waist circumference, and hip circumference). Height and weight measurements were carried out without heavy clothing or shoes. The BMI was calculated based on the height measurements in metres and body mass measurements in kilograms [kg/m2]. Waist circumference was measured over the unclothed abdomen at the level of the midpoint between the lower margin of the ribs and the anterior superior iliac crest spine. The hip circumference was measured at the level of the greater trochanters. In all enrolled patients serum samples were obtained after 12 h or more of fasting. On the same day, measurements of blood pressure, heart rate, and fasting glucose were taken as well as lipid profile samples. Additionally, in a subgroup of patients (LIPIDOGEN2015 sub-study), saliva samples for DNA isolation and additional blood samples for measurement of glycated haemoglobin concentration, oxidative stress parameters, autoantibody levels, inflammatory cytokine profile, and apolipoprotein profile were collected (Figure 1).
Clinical chemistry
The collected blood samples were transferred in cooled containers to a central laboratory (Silesian Analytical Laboratories – SLA in Katowice, Poland). The laboratory meets appropriate International Organisation for Standardisation (ISO) and Good Laboratory Practice (GLP) standards and also regularly undergoes internal, external, and international audits. Measurements of total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) (with direct immunological measurement) were performed and carried out using the same methodology and the same Siemens Advia 1800 analyser and Siemens reagents (Munich, Germany), within 12 h of obtaining the blood sample. Fasting glycaemia was measured using Bionime glucometers (Taichung City, Taiwan) and Rightest strip tests (Taichung City, Taiwan). HbA1c was assessed using high-performance liquid chromatography (HPLC) performed by Variant II Turbo (Bio-Rad, Hercules, California, USA).
Oxidative stress measurement
For the evaluation of oxidative stress intensity, the indices of free-radical damage to lipids and proteins, enzymatic and non-enzymatic antioxidant system parameters in serum, and erythrocytes were measured. In serum, indices of free-radical damage to lipids and proteins include the following: total oxidant capacity (TOC) [30], lipid hydroperoxides (LPH) [31], lipofuscin (LPS) [32], and protein thiol groups [33] (PSH), and malondialdehyde (MDA) [34] concentration. The activity of serum superoxide dismutase (SOD) [35] was also determined. Additionally, the parameters of a non-enzymatic antioxidant system, such as total antioxidant status (TAS) [36], the concentration of ceruloplasmin (CER) [37], albumin, bilirubin, and uric acid (UA) were determined. Based on TOC and TAS values, the oxidative stress index (OSI) was calculated. In erythrocytes, the indices of free-radical damage to lipids and proteins, such as LPS and MDA, were determined. The analysis included also the activities of SOD [35], catalase (CAT) [38], and the enzymes related to glutathione metabolism, such as glutathione peroxidase (GPx) [39], glutathione-S-transferase (GST) [40], and glutathione reductase (GR) [37].
Autoantibody assessment
Anti-nuclear (ANA) antibodies were detected by indirect immunofluorescence on human laryngeal carcinoma cells (HEp-2) with commercially available Euroimmun Medizinische Labordiagnostika AG (Lübeck, Germany) test kits (Mosaic Basic Profiles 3; catalogue number FC 1800-2010-3). Sample incubations were carried out manually, according to the instructions included in the test kit. The results were evaluated on a EUROstar III fluorescence microscope (CarlZeiss Oberkochen, Germany). The test result includes a qualitative assessment of the presence of ANA antibodies, estimation of antibody titre, and determination of the characteristic pattern according to the ICAP (International Consensus on Antinuclear Antibody Pattern) nomenclature. The concentrations of anti-DFS70, CCP, and CEP1-α antibodies were determined by ELISA method. We used commercially available Euroimmun Medizinische Labordiagnostika AG (Euroimmun AG, Lübeck, Germany) test kits. Incubation were performed automatically with a Euroimmun Analyzer I (Euroimmun AG, Lübeck, Germany) according to the instructions included in the test kit.
Apolipoproteins and inflammatory markers
We also assumed that for the evaluation of inflammatory response and apolipoprotein levels in serum the Bio-Plex Pro Human Apolipoprotein Assay Panel and Bio-Plex Pro Human Inflammation Panel (Bio-Rad, Hercules, California, USA) will be used. Concentrations of apolipoproteins, such as Apo A1, Apo A2, Apo B, Apo C1, Apo C3, Apo D, Apo E, Apo H, and Apo J, will be determined. The analysis of biomarkers of inflammation will include: C-reative protein (CRP), Treg cytokines (IL-2, IL-10, IL-12, IL-19, IL-20, IL-22, IL-26, IL-27, IL-35, and interferon (IFN)-λ2 and IFN-λ1), matrix metalloproteinases (MMP-1, MMP-2, MMP-3), tumour necrosis factor (TNF) superfamily proteins, and IFN family proteins (APRIL/TNFSF13, BAFF/TNFSF13B, sCD30/TNFRSF8, sCD163, chitinase-3-like 1, gp130/sIL-6Rβ, IFN-α2, IFN-β, IFN-γ, sIL-6Rα, IL-8, IL-11, IL-32, IL-34, LIGHT/TNFSF14, osteocalcin, osteopontin, pentraxin-3, sTNF-R1, sTNF-R2, TSLP, and TWEAK/TNFSF12).
Genetic analyses
Two-millilitre saliva samples were collected according to the manufacturer’s instructions for the Oragene-DNA / OG-500 kit (DNA Genotek, Ottawa, Canada). Saliva was collected and banked according to the manufacturer’s instructions until DNA isolation. Isolation of banked saliva will be carried out using the PrepIT-L2P kit (DNA Genotek, Ottawa, Canada) according to the manufacturer’s protocol. In the next step, quantitative and qualitative analyses will be carried out by a combination of agarose gel electrophoresis and spectrophotometry (concentration measurement and absorbance ratio 260/280).
In order to identify gene variants related to lipid disorders and/or associated with susceptibility to metabolic syndrome, hypertension, diabetes mellitus, CAD, and autoimmune diseases we will use custom panels including known and candidate genes associated with the above-mentioned diseases. The reaction will be performed on the MiSeq sequencer (Illumina, San Diego, USA) based on sequencing technologies by synthesis. The results from the sequencing will be analysed using the commercial software Variant Studio v3.0 (Illumina, San Diego, USA) as well as online tools.
Follow-up data
Follow-up data on all-cause mortality, cardiovascular outcomes, and hospitalisations will be obtained from the National Health Fund, which is an obligatory nationwide health insurance institution.
Ethical approval
All individuals signed an informed consent form to participate in the study and gave their permission to use anonymous questionnaire data, the results of their laboratory tests, and secured biological material for the purpose of statistical and scientific studies. The LIPIDOGRAM2015 and LIPIDOGEN2015 studies received a positive opinion from the Bioethical Commission of the Chamber of Physicians on 02/12/2015 (No. K.B.Cz.-0018/2015) and conform to the principles outlined in the Declaration of Helsinki.
Statistical analysis
Analysis will be performed with R [41] and Statistica 13.3 software (StatSoft, Tulsa). Data will be expressed as mean ± SD (for normal distribution) and median (nonparametric distribution) for continuous variables, and as a percentage for categorical variables. Univariate comparison of markers related to autoimmune diseases according to clinical variables will be performed using the U-Mann-Whitney method for nonparametric variables or χ2 test/Fisher exact test where appropriate. Spearman correlations for categorical variables will be performed to evaluate relationships between autoimmune diseases and laboratory tests as well as other parameters. Uni- and multivariate logistic regression analyses will be used to determine the association between clinical and genetic variables and abnormal parameters of oxidative stress. Additionally, due to the large number of variables, dimensionality reduction techniques might be used in some analyses. A two-sided p < 0.05 will be considered statistically significant, and in case of genetic analysis the Bonferroni correction or false discovery rate will be applied.
Results
The study summary for patients
At the conclusion of the LIPIDOGRAM2015 and LIPIDOGEN2015 studies, each enrolled participant received his/her test results with an interpretation made by a physician-investigator, along with a non-promotional book promoting healthy lifestyle.
Baseline characteristics
The clinical characteristics of the LIPIDOGRAM2015 and LIPIDOGEN2015 study cohort will reflect the frequency of occurrence of cardiovascular risk factors and concomitant diseases and will include all of the variables listed in Table II. Additionally, the lipid profile and glucose level will be presented. For the LIPIDOGEN2015 sub-study, HbA1c data will be available (Table III).
Table II
Anthropometric and clinical data obtained from enrolled patients of the LIPIDOGRAM2015 and LIPIDOGEN2015 studies
Table III
Laboratory tests performed in the LIPIDOGRAM2015 and LIPIDOGEN2015 studies
Autoantibody assessment
The occurrence of tested autoantibodies will be assessed in the context of concomitant diseases oxidative stress parameters and genes related to autoimmune disorders, as well as long-term prognosis.
Oxidative stress
Parameters of oxidative stress will be correlated with baseline clinical characteristics as well as the concentrations of different fractions of apolipoproteins, markers of inflammation, and the presence of autoantibodies.
Apolipoprotein and inflammatory cytokine profile
We will aim to correlate apolipoprotein and inflammatory cytokine profiles with the presence of atherosclerotic diseases, treatment of dyslipidaemia, as well as long-term prognosis in patients with hypertension, diabetes, etc.
Genetic data
Mutations and common variants allegedly conferring susceptibility to CVD and cardiometabolic diseases will be correlated with the presence of concomitant diseases. We will also derive a genetic risk score for CVD and cardiometabolic diseases. Additionally, we will make clinical diagnoses of familial hypercholesterolaemia by identifying causative variants.
Discussion
LIPIDOGRAM2015 and LIPIDOGEN2015 assess the prevalence of dyslipidaemia and other cardiovascular risk factors in 2015/2016 as a follow-up to the previous LIPIDOGRAM cohort studies started in 2004, consequently giving the perspective of CVD risk factor distribution changes in a more than 10-year perspective [5–7]. In many countries a worrisome trend towards more frequent occurrence of dyslipidaemia, obesity, and diabetes is being observed [42, 43]. A comparison of data from LIPIDOGRAM2015 with previous editions will enable us to analyse those trends; we will also be able to estimate how many patients reach LDL targets in primary and secondary prevention of cardiovascular disease, and whether it has changed over the last decade. In populations from various countries it was shown that no more than 30% of patients reach LDL target levels both in primary and secondary prevention of CAD [44–47]. This type of epidemiological data may influence dyslipidaemia treatment guidelines for PHC, but are also of great importance not only for Polish family physicians, but also for cardiologists to whom patients are referred for consultations [48]. Furthermore, public health specialists, medical care administrators, and the public health care payer (NFZ – Polish National Health Fund) managing and financing the health care system will also benefit. Health politicians who plan changes in the health care system in Poland will have the ability to make use of project’s results.
The LIPIDOGEN2015 sub-study also allows us to assess the apolipoprotein and inflammatory cytokine profile, enzymatic and non-enzymatic parameters associated with oxidative stress, selected parameters of the immune response, prevalence of antinuclear autoantibodies and antibodies against citrullinated protein/peptide antigens (ACPA), as well as genetics of lipid disorders and susceptibility to CVD and cardiometabolic and atherosclerotic diseases in a large Polish population. Low-grade inflammation and oxidative stress play a role in the pathogenesis of diabetes mellitus and coronary artery disease [49, 50]. The LIPIDOGEN sub-study, will enable us to analyse inflammatory cytokine profiles and oxidative stress in the context of ASCVD and diabetes mellitus as well as the concomitant diseases.
Autoantibodies are an essential finding in rheumatological diseases, which substantially increase the risk of coronary artery disease in affected individuals. ANA are, however, present in up to 30% of patients without any diagnosed rheumatological disease [51, 52], and the presence of ANA and CCP has been shown to be associated with CV events in patients with and without rheumatological diseases [53]. The presence of ANA corelates with markers of inflammation, but not with classical CV risk factors, which might implicate different pathophysiological pathways in the progression of atherosclerosis mediated, at least in part, by ANA [51, 52]. Based on our best knowledge, such large cohort population studies, like LIPIDOGEN2015 sub-study, for the prevalence of ANA, DFS70, CCP, and CEP-1α antibodies have not yet been carried out in the Polish population. We hope that ours will also be of importance in planning studies assessing causal relationship between autoimmunological diseases, inflammation, and atherosclerosis.
In addition, 4-year follow-up data on mortality, hospitalisations, occurrence of cardiovascular outcomes, including acute coronary syndromes, and stroke will allow us to draw conclusions on factors affecting prognosis in the LIPIDOGRAM population. In the LIPIDOGEN2015 sub-study we will be able to include in the survival analysis unique laboratory data on apolipoprotein and cytokine profiles, oxidative stress parameters, and the presence of ANA, DFS70, CCP, and CEP-1α antibodies. Therefore, we believe that the results of the study will be not only of national, but also of international importance. They will allow a comparison of the situation in Poland with other countries in Europe and around the world [54, 55].
The main limitation of this study comes from its observational design; therefore, definite causal inferences will not be possible. Patient recruitment through primary care channels may lead to overrepresentation of patients burdened with concomitant diseases; however, the inclusion criteria were very broad. From previous editions of the LIPIDOGRAM study, we know that the prevalence of classical risk factors is similar to that of other epidemiological studies carried out in Poland [5–7].
In conclusion, the LIPIDOGRAM2015 and LIPIDOGEN2015 studies are the first and among the most important evaluating the distribution of cardiovascular risk factors in consecutive patients in a primary care setting. These studies also assesses the relationship between inflammation, oxidative stress, and mutations in genes coding proteins involved in lipid metabolism as well as genes conferring susceptibility to CVD and cardiometabolic diseases. These studies might make a critical contribution regarding the role of gene-environment interaction in the development of CV and related diseases.