Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ONCOLOGY / BASIC RESEARCH
ENTPD1-AS1 promotes gastric cancer by activating the WNT signaling pathway through the FTO/WIF1 axis and interaction with SRSF1
1
Department of Gastrointestinal and Hepatobiliary Surgery, the Affiliated Hospital of Hangzhou Normal University, 126 Wenzhou Road, Hangzhou, Zhejiang, 310000, China
2
Department of Surgery, School of Clinical Medicine, Hangzhou Normal University, Hangzhou, 311121, China
3
Department of General Surgery, the Affiliated Hospital of Hangzhou Normal University, 126 Wenzhou Road, Hangzhou, Zhejiang, 310000, China
Submission date: 2025-03-17
Final revision date: 2025-07-10
Acceptance date: 2025-07-23
Online publication date: 2025-09-20
Corresponding author
Zhijian Pan
Department of General Surgery Affiliated Hospital of Hangzhou Normal University 126 Wenzhou Road Hangzhou Zhejiang, 310000, China
Department of General Surgery Affiliated Hospital of Hangzhou Normal University 126 Wenzhou Road Hangzhou Zhejiang, 310000, China
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Gastric cancer (GC) remains a major global health burden. Emerging evidence highlights the critical regulatory roles of long noncoding RNAs (lncRNAs) in GC pathogenesis. The purpose of this study was to explore the role and potential regulatory mechanisms of a lncRNA, ENTPD1-AS1, in GC.
Material and methods:
Functional assays (CCK-8, colony formation, wound healing and Transwell assays) were performed in AGS and MKN45 cells following ENTPD1-AS1/SRSF1 knockdown or WIF1 overexpression. Targets were screened via RNA-seq and bioinformatics (UALCAN and SRAMP databases). The m6A modification levels were assessed by MeRIP-qPCR, and molecular interactions were validated by RNA immunoprecipitation (RIP) and immunofluorescence (IF) assays.
Results:
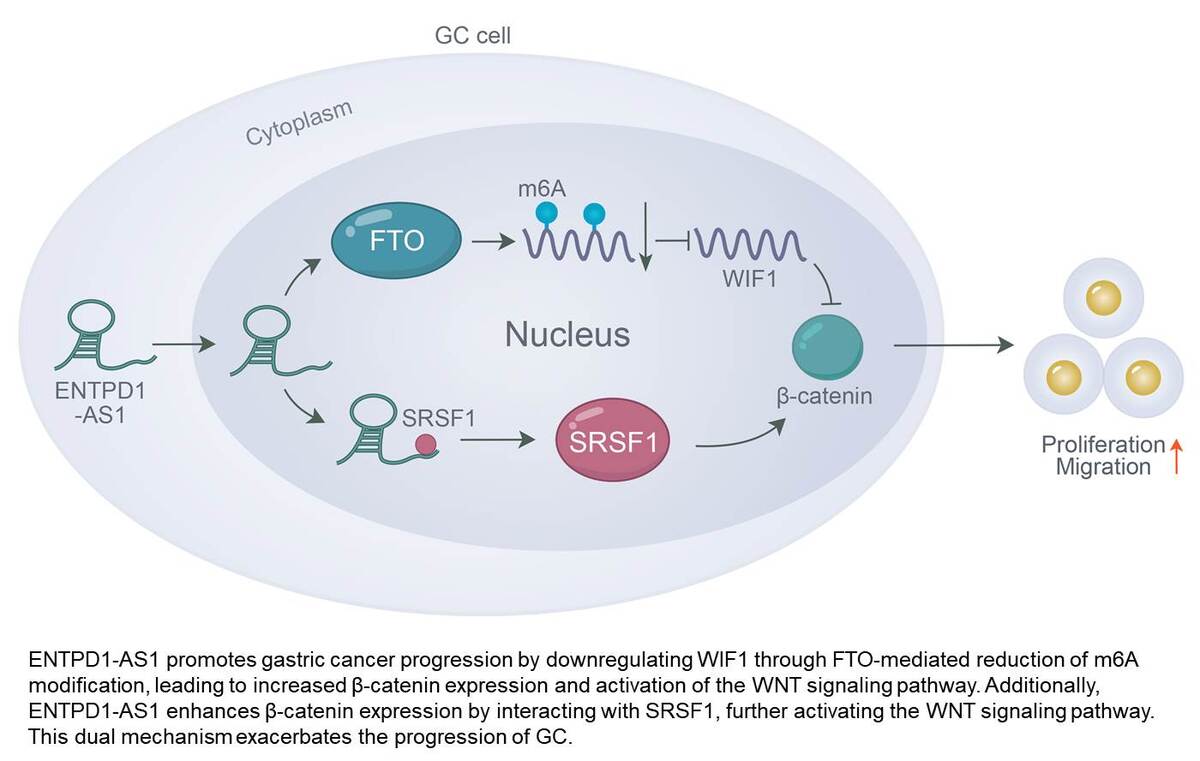
Knockdown of ENTPD1-AS1 & SRSF1 and overexpression of WIF1 inhibited the proliferation, migration, and invasion of GC cells. Inhibition of ENTPD1-AS1 also increased the m6A modification levels in GC cells. Mechanistically, ENTPD1-AS1 downregulated WIF1 through FTO-mediated low levels of m6A modification to promote β-catenin expression, thereby activating the WNT signaling pathway and exacerbating GC. Additionally, ENTPD1-AS1 promoted GC progression by enhancing β-catenin expression through interaction with SRSF1 to activate the WNT signaling pathway.
Conclusions:
In summary, the research highlights the significance of the ENTPD1-AS1/FTO/WIF1 and ENTPD1-AS1/SRSF1 axes in collaboratively activating the WNT signaling pathway. These findings identify ENTPD1-AS1 as a potential therapeutic target for GC.
Gastric cancer (GC) remains a major global health burden. Emerging evidence highlights the critical regulatory roles of long noncoding RNAs (lncRNAs) in GC pathogenesis. The purpose of this study was to explore the role and potential regulatory mechanisms of a lncRNA, ENTPD1-AS1, in GC.
Material and methods:
Functional assays (CCK-8, colony formation, wound healing and Transwell assays) were performed in AGS and MKN45 cells following ENTPD1-AS1/SRSF1 knockdown or WIF1 overexpression. Targets were screened via RNA-seq and bioinformatics (UALCAN and SRAMP databases). The m6A modification levels were assessed by MeRIP-qPCR, and molecular interactions were validated by RNA immunoprecipitation (RIP) and immunofluorescence (IF) assays.
Results:
Knockdown of ENTPD1-AS1 & SRSF1 and overexpression of WIF1 inhibited the proliferation, migration, and invasion of GC cells. Inhibition of ENTPD1-AS1 also increased the m6A modification levels in GC cells. Mechanistically, ENTPD1-AS1 downregulated WIF1 through FTO-mediated low levels of m6A modification to promote β-catenin expression, thereby activating the WNT signaling pathway and exacerbating GC. Additionally, ENTPD1-AS1 promoted GC progression by enhancing β-catenin expression through interaction with SRSF1 to activate the WNT signaling pathway.
Conclusions:
In summary, the research highlights the significance of the ENTPD1-AS1/FTO/WIF1 and ENTPD1-AS1/SRSF1 axes in collaboratively activating the WNT signaling pathway. These findings identify ENTPD1-AS1 as a potential therapeutic target for GC.
REFERENCES (35)
1.
Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol 2020; 18: 534-42.
2.
Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci 2020; 21: 4012.
3.
Petryszyn P, Chapelle N, Matysiak-Budnik T. Gastric cancer: Where are we heading? Dig Dis 2020; 38: 280-5.
4.
Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol 2023; 16: 57.
5.
Tan Z. Recent advances in the surgical treatment of advanced gastric cancer: a review. Med Sci Monit 2019; 25: 3537-41.
6.
Xia JY, Aadam AA. Advances in screening and detection of gastric cancer. J Surg Oncol 2022; 125: 1104-9.
7.
Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev 2020; 39: 1179-203.
8.
Necula L, Matei L, Dragu D, et al. Recent advances in gastric cancer early diagnosis. World J Gastroenterol 2019; 25: 2029-44.
9.
Chen Z, Li Y, Tan B, et al. Long non-coding RNA ASNR targeting miR-519e-5p promotes gastric cancer development by regulating FGFR2. Fron Cell Dev Biol 2021; 9: 679176.
10.
Wang S, Zhu W, Qiu J, Chen F. lncRNA SNHG4 promotes cell proliferation, migration, invasion and the epithelial-mesenchymal transition process via sponging miR-204-5p in gastric cancer. Mol Med Rep 2021; 23: 85.
11.
Tang LH, Ye PC, Yao L, et al. LINC01268 promotes epithelial-mesenchymal transition, invasion and metastasis of gastric cancer via the PI3K/Akt signaling pathway and targeting MARCKS. World J Gastrointest Oncology 2023; 15: 1366-83.
12.
He C, Qi W, Wang Z. Effect and mechanism of downregulating the long-chain noncoding RNA TM4SF1-AS1 on the proliferation, apoptosis and invasion of gastric cancer cells. World J Surg Oncol 2021; 19: 226.
13.
Jin Y, Jiang D. GATA6-AS1 via sponging miR-543 to regulate PTEN/AKT signaling axis suppresses cell proliferation and migration in gastric cancer. Mediators Inflamm 2023; 2023: 9340499.
14.
Yuan HM, Pu XF, Wu H, Wu C. ENTPD1-AS1-miR-144-3p-mediated high expression of COL5A2 correlates with poor prognosis and macrophage infiltration in gastric cancer. World J Gastrointest Oncol 2023; 15: 1182-99.
15.
Sun T, Wu R, Ming L. The role of m6A RNA methylation in cancer. Biomed Pharmacother 2019; 112: 108613.
16.
Huang H, Weng H, Chen J. m6A Modification in coding and non-coding RNAs: roles and therapeutic implications in cancer. Cancer Cell 2020; 37: 270-88.
17.
Zhang N, Wen K. The role of lncRNA binding to RNA binding proteins to regulate mRNA stability in cancer progression and drug resistance mechanisms (Review). Oncol Rep 2024; 52: 142.
18.
Zhou X, Xiao L, Meng F, et al. GAS6-AS1 drives bladder cancer progression by increasing MMP7 expression in a ceRNA- and RBP-dependent manner. Transl Oncol 2024; 48: 102065.
19.
Tao W, Ma J, Zheng J, et al. Silencing SCAMP1-TV2 inhibited the malignant biological behaviors of breast cancer cells by interaction with PUM2 to facilitate INSM1 mRNA degradation. Front Oncol 2020; 10: 613.
20.
Koni M, Pinnarò V, Brizzi MF. The Wnt signalling pathway: a tailored target in cancer. Int J Mol Sci 2020; 21: 7697.
21.
Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene 2017; 36: 1461-73.
22.
Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci 2003; 116 (Pt 13): 2627-34.
23.
Catana CS, Crișan CA, Opre D, Berindan-Neagoe I. Implications of long non-coding RNAs in age-altered proteostasis. Aging Dis 2020; 11: 692-704.
24.
Fu Y, Huang B, Shi Z, et al. SRSF1 and SRSF9 RNA binding proteins promote Wnt signalling-mediated tumorigenesis by enhancing -catenin biosynthesis. EMBO Mol Med 2013; 5: 737-50.
25.
Zhang H, Wang J, Xun W, Wang J, Song W, Wang X. Long non-coding RNA PTCSC3 inhibits human oral cancer cell proliferation by inducing apoptosis and autophagy. Arch Med Sci 2020; 17: 492-9.
26.
Huang HT, Xu YM, Ding SG, et al. The novel lncRNA PTTG3P is downregulated and predicts poor prognosis in non-small cell lung cancer. Arch Med Sci 2020; 16: 931-40.
27.
Zhao J, Li X, Fu L, Zhang N, Yang J, Cai J. lncRNA LIFR AS1 inhibits gastric carcinoma cell proliferation, migration and invasion by sponging miR 4698. Mol Med Rep 2021; 23: 153.
28.
Liu G, Jiang Z, Qiao M, Wang F. Lnc-GIHCG promotes cell proliferation and migration in gastric cancer through miR-1281 adsorption. Mol Genet Genomic Med 2019; 7: e711.
29.
He ZC, Yang F, Guo LL, Wei Z, Dong X. LncRNA TP73-AS1 promotes the development of Epstein-Barr virus associated gastric cancer by recruiting PRC2 complex to regulate WIF1 methylation. Cell Signal 2021: 110094.
30.
Zhang QC. METTL3 is aberrantly expressed in endometriosis and suppresses proliferation, invasion, and migration of endometrial stromal cells. Kaohsiung J Med Sci 2023; 39: 266-77.
31.
Fu Q, Tan X, Tang H, Liu J. CCL21 activation of the MALAT1/SRSF1/mTOR axis underpins the development of gastric carcinoma. J Transl Med 2021; 19: 210.
32.
Wu ZH, Liu CC, Zhou YQ, Hu LN, Guo WJ. OnclncRNA-626 promotes malignancy of gastric cancer via inactivated the p53 pathway through interacting with SRSF1. Am J Cancer Res 2019; 9: 2249-63.
33.
Zhang C, Yang Y, Yi L, Paizula X, Xu W, Wu X. HOXD Antisense growth-associated long noncoding RNA promotes triple-negative breast cancer progression by activating Wnt signaling pathway. J Breast Cancer 2021; 24: 315-29.
34.
Wang S, Sun Z, Lei Z, Zhang HT. RNA-binding proteins and cancer metastasis. Semin Cancer Biol 2022; 86 (Pt 2): 748-68.
35.
Yao ZT, Yang YM, Sun MM, et al. New insights into the interplay between long non-coding RNAs and RNA-binding proteins in cancer. Cancer Commun (Lond) 2022; 42: 117-40.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.